Importance of Wetlands Management for West Nile Virus Circulation Risk, Camargue, Southern France
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area

2.2. Field Sampling and Laboratory Analysis
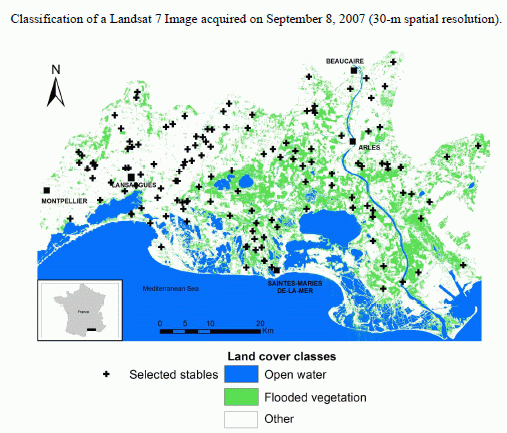
2.3. Environmental Data
| Groups | Dates of Images | |
|---|---|---|
| Very low level of mosquito activity = “NOT” | 2006: | 10 December |
| 26 December | ||
| 2007: | 11 January | |
| 27 January | ||
| 13 December | ||
| 2008: | 15 February | |
| Medium level of mosquito activity = “END” | 2007: | 16 March |
| 17 April | ||
| 2008: | 2 March | |
| 22 June | ||
| 8 July | ||
| High level of mosquito activity = “EPI” | 2006: | 20 August |
| 2007: | 8 September | |
| 24 September | ||
| 2008: | 9 August | |
2.4. Statistical Analysis
- (1)
- The log-transformed mean percentages of the area corresponding to open water or flooded vegetation in buffers around the stables, during the three time periods NOT, END and EPI (6 variables).
- (2)
- The differences between these percentages between the three periods: NOT-END, END-EPI and NOT-EPI (6 variables). For example, for a given location, END-EPI for open water represents the mean percentage of the area of open water during the END period minus the mean percentage of the open water area during the EPI period and expresses the variation in open water areas between the endemic and epidemic periods, within a buffer of 2 km radius around the stables. These differences were categorized in tertiles.
3. Results and Discussion
3.1. Results
3.1.1. Serological Results

3.1.2. Univariate Analysis

| Variable | Mean (Min; Max) | Adjusted Odds-Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|---|
| Mean percentage (%) of open water area a: | ||||
| NOT | 5.025 (0.0; 52.70) | 2.07 | [1.47–2.92] | <0.001 |
| END | 4.962 (0.0; 50.83) | 2.16 | [1.53–3.05] | <0.001 |
| EPI | 4.083 (0.0; 50.67) | 1.91 | [1.35–2.7] | <0.001 |
| Mean % of flooded vegetation area a: | ||||
| NOT | 8.831 (0.88; 31.84) | 2.96 | [1.46–6.02] | 0.003 |
| END | 8.170 (0.09; 35.98) | 3.35 | [1.94–5.79] | <0.001 |
| EPI | 21.612 (1.06; 64.61) | 2.44 | [1.42–4.2] | 0.001 |
| Differences in mean % of open water area (e.g.,: NOT-END = mean% NOT − mean% END) : | ||||
| NOT-END | 0.064 (−3.70; 3.88) | |||
| [−3.70; 0.01] | NS | [0.29–0.83] | 0.008 | |
| [0.01; 0.14] | 0.49 | |||
| [0.14; 3.88] | NS | |||
| END-EPI | 0.878 (−1.03; 8.01) | |||
| [−1.03; 0.0] | NS | |||
| [0.0; 0.88] | NS | |||
| [0.88; 8.01] | 3.83 | [1.71–8.56] | 0.001 | |
| NOT-EPI | 0.943 (−1.73; 10.50) | |||
| [−1.73; 0.04] | NS | |||
| [0.04; 0.78] | NS | |||
| [0.78; 10.50] | 2.15 | [1.35–3.43] | 0.001 | |
| Differences in mean % of flooded vegetation area: | ||||
| NOT-END | 0.661 (−13.15; 11.92) | |||
| [−13.15; −0.08] | NS | [0.38–0.9] | 0.015 | |
| [−0.08; 2.88] | 0.59 | |||
| [2.88; 11.92] | NS | |||
| END-EPI | −13.440 (−46.43; −1.06) | |||
| [−46.43; −17.55] | NS | |||
| [−17.55; −5.11] | 0.49 | [0.31–0.79] | 0.003 | |
| [−5.11; −1.06] | 0.58 | [0.38–0.88] | 0.011 | |
| NOT-EPI | −5.747 (−48.01; 3.97) | |||
| [−48.01; −16.97] | NS | |||
| [−16.97; −2.34] | NS | |||
| [−2.34; 3.97] | 0.45 | [0.27–0.76] | 0.003 | |
| Variable | Counts | Categories | % Seropositive | Adjusted Odds-Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|---|---|---|
| Horse gender | 1157 a | |||||
| 133 | Stallion | 0.4 | NS | |||
| 673 | Gelding | 9.0 | 4.71 | [1.88–11.82] | <0.001 | |
| 351 | Mare | 2.9 | 2.67 | [1.02–6.99] | 0.046 | |
| Horse breed | 1159 a | |||||
| 405 | Other | 2.8 | NS | |||
| 632 | Camargue | 9.1 | 2.21 | |||
| 122 | Iberic | 0.4 | NS | [1.45–3.37] | <0.001 | |
| Horse activity | 1156 a | |||||
| 297 | Other | 2.9 | NS | |||
| 179 | Breeding | 2.0 | NS | |||
| 276 | Livestock cutting | 4.3 | 2.11 | [1.29–3.48] | 0.003 | |
| 404 | Riding school | 3.1 | NS | |||
| Horse birth date | 1136 a | |||||
| 185 | ≤1990 | 3.6 | 24.85 | [3.35–184.39] [1.72–92.48] [1.52–81.74] | <0.001 | |
| 413 | [1991–1997] | 4.4 | 12.61 | <0.001 | ||
| 446 | [1998–2004] | 4.2 | 11.13 | <0.001 | ||
| 92 | ≥2005 | 0.1 | NS | |||
| Date of horse acquisition | 1122 a | |||||
| 384 | ≤1999 | 7.0 | NS | |||
| 422 | [2000–2004] | 4.0 | 0.45 | [0.3–0.67] | <0.001 | |
| 316 | ≥2005 | 1.2 | 0.16 | [0.09–0.3] | <0.001 |
3.1.3. Multivariate Analysis
- (1)
- Age, breed, date of acquisition in the stable, activity,
- (2)
- Mean percentage of open water area during the EPI period and mean percentages of flooded vegetation during the NOT and END periods,
- (3)
- All the differences in open water and flooded vegetation areas except the difference in flooded vegetation between the NOT and EPI periods.
| Variable | Adjusted Odds-ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| Horse birth date: | |||
| ≤1990 | 1 | ||
| [1991–1997] | 0.65 | 0.36–1.17 | 0.147 |
| [1998–2004] | 0.97 | 0.49–1.91 | 0.921 |
| ≤2005 | 0.09 | 0.01–0.93 | 0.043 |
| Date of horse acquisition: | |||
| ≤1999 | 1 | ||
| [2000–2004] | 0.50 | 0.28–0.87 | 0.014 |
| ≥2005 | 0.27 | 0.12–0.62 | 0.002 |
| Differences in mean % of open water area | (2 km buffers) | ||
| NOT-END | |||
| [−3.70; 0.01] | 1 | ||
| [0.01; 0.14] | 1.78 | 0.62–5.13 | 0.285 |
| [0.14; 3.88] | 2.33 | 1.03–5.28 | 0.042 |
| END-EPI | |||
| [−1.03; 0.0] | 1 | ||
| [0.0; 0.88] | 1.10 | 0.33–3.61 | 0.878 |
| [0.88; 8.01] | 5.53 | 1.53–20.01 | 0.009 |
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ulbert, S. West Nile virus: The complex biology of an emerging pathogen. Intervirology 2011, 54, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Hendrikx, P.; Sabatier, P. West Nile virus outbreak detection using syndromic monitoring in horses. Vector Borne Zoonotic Dis. 2007, 7, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef] [PubMed]
- ECDC. West Nile Fever Maps. Available online: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps (accessed on 10 June 2014).
- Pradier, S.; Lecollinet, S.; Leblond, A. West Nile virus epidemiology and factors triggering change in its distribution in Europe. Rev. Sci. Tech. 2012, 31, 829–844. [Google Scholar] [PubMed]
- Calzolari, M.; Monaco, F.; Montarsi, F.; Bonilauri, P.; Ravagnan, S.; Bellini, R.; Cattoli, G.; Cordioli, P.; Cazzin, S.; Pinoni, C.; et al. New incursions of West Nile virus lineage 2 in Italy in 2013: The value of the entomological surveillance as early warning system. Vet. Ital. 2013, 49, 315–319. [Google Scholar] [PubMed]
- Eloit, M. West Nile virus, horses—France: OIE. ProMed. 2006. archive number: 20061010.2903. Available online: http://www.promedmail.org (accessed on 10 June 2014).
- Balanca, G.; Gaidet, N.; Savini, G.; Vollot, B.; Foucart, A.; Reiter, P.; Boutonnier, A.; Lelli, R.; Monicat, F. Low West Nile virus circulation in wild birds in an area of recurring outbreaks in Southern France. Vector Borne Zoonotic Dis. 2009, 9, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, M.; Lecollinet, S.; Jourdain, E.; Thomas, F.; Blanchon, T.; Arnal, A.; Lowenski, S.; Gauthier-Clerc, M. Recent circulation of West Nile virus and potentially other closely related flaviviruses in Southern France. Vector Borne Zoonotic Dis. 2013, 13, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Monaco, F.; Savini, G.; Calistri, P.; Polci, A.; Pinoni, C.; Bruno, R.; Lelli, R. 2009 West Nile disease epidemic in Italy: First evidence of overwintering in Western Europe? Res. Vet. Sci. 2011, 91, 321–326. [Google Scholar] [CrossRef]
- Balenghien, T.; Vazeille, M.; Grandadam, M.; Schaffner, F.; Zeller, H.; Reiter, P.; Sabatier, P.; Fouque, F.; Bicout, D.J. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2008, 8, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Poncon, N.; Toty, C.; L’Ambert, G.; le Goff, G.; Brengues, C.; Schaffner, F.; Fontenille, D. Population dynamics of pest mosquitoes and potential malaria and West Nile virus vectors in relation to climatic factors and human activities in the Camargue, France. Med. Vet. Entomol. 2007, 21, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ponçon, N.; Tran, A.; Toty, C.; Luty, A.J.F.; Fontenille, D. A quantitative risk assessment approach for mosquito-borne diseases: Malaria re-emergence in southern France. Malar. J. 2008, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Mouchet, J.; Rageau, J.; Hannoun, C.; Joubert, L.; Oudar, J.; Beytout, D. Epidemiology of the West Nile virus: Study of an outbreak in Camargue. II. Outline of the physical, biological and human environment. Ann. Inst. Pasteur. (Paris) 1968, 114, 521–538. (In French) [Google Scholar]
- Isenmann, P. Oiseaux de Camargue; Société d’Etudes Ornithologiques: Brunoy, France, 1993; p. 158. (In French) [Google Scholar]
- Poncon, N.; Balenghien, T.; Toty, C.; Baptiste Ferre, J.; Thomas, C.; Dervieux, A.; L’Ambert, G.; Schaffner, F.; Bardin, O.; Fontenille, D. Effects of local anthropogenic changes on potential malaria vector Anopheles hyrcanus and West Nile virus vector Culex modestus, Camargue, France. Emerg. Infect. Dis. 2007, 13, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Sandoz, A.; Lefebvre, G.; Zeller, H.; Bicout, D.J. Remote sensing based identification of environmental risk factors associated with West Nile disease in horses in Camargue, France. Prev. Vet. Med. 2007, 79, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Zientara, S.; Chadoeuf, J.; Comby, N.; Heng, M.A.; Sabatier, P. Prévalence de l’infection par le virus West Nile chez le cheval en Camargue en 2001. Rev. Med. Vet. 2005, 156, 77–84. (In French) [Google Scholar]
- USGS. Landsat 7 Archive Earth Explorer. Available online: http://glovis.usgs.gov (accessed on 28 January 2014).
- Balenghien, T.; Fouque, F.; Sabatier, P.; Bicout, D.J. Horse-, bird-, and human-seeking behavior and seasonal abundance of mosquitoes in a West Nile virus focus of southern France. J. Med. Entomol. 2006, 43, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer Verlag: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; AVC Inc.: Charlottetown, Canada, 2003; p. 865. [Google Scholar]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Pfeiffer, D.U.; Robinson, T.P.; Stevenson, M.; Stevens, K.B.; Rogers, D.J.; Clements, A.C.A. Spatial Analysis in Epidemiology; Oxford University Press: New York, NY, USA, 2008; p. 162. [Google Scholar]
- Guis, H.; Tran, A.; de la Rocque, S.; Baldet, T.; Gerbier, G.; Barragué, B.; Biteau-Coroller, F.; Roger, F.; Viel, J.-F.; Mauny, F. Use of high spatial resolution satellite imagery to characterize landscapes at risk for bluetongue. Vet. Res. 2007, 38, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Hay, S.I.; Bundy, D.A.P. Tools from ecology: Useful for evaluating infection risk models? Trends Parasitol. 2002, 18, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.R. Is global warming harmful to health? Sci. Am. 2000, 283, 50–57. [Google Scholar]
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-Induced amplification and epidemic transmission of West Nile virus in southern Florida. J. Med. Entomol. 2005, 42, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Mongoh, M.N.; Khaitsa, M.L.; Dyer, N.W. Environmental and ecological determinants of West Nile virus occurrence in horses in North Dakota, 2002. Epidemiol. Infect. 2007, 135, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Epp, T.Y.; Waldner, C.; Berke, O. Predictive risk mapping of West Nile virus (WNV) infection in Saskatchewan horses. Can. J. Vet. Res. 2011, 75, 161–170. [Google Scholar] [PubMed]
- Ward, M.P.; Wittich, C.A.; Fosgate, G.; Srinivasan, R. Environmental risk factors for equine West Nile virus disease cases in Texas. Vet. Res. Commun. 2009, 33, 461–471. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pradier, S.; Sandoz, A.; Paul, M.C.; Lefebvre, G.; Tran, A.; Maingault, J.; Lecollinet, S.; Leblond, A. Importance of Wetlands Management for West Nile Virus Circulation Risk, Camargue, Southern France. Int. J. Environ. Res. Public Health 2014, 11, 7740-7754. https://doi.org/10.3390/ijerph110807740
Pradier S, Sandoz A, Paul MC, Lefebvre G, Tran A, Maingault J, Lecollinet S, Leblond A. Importance of Wetlands Management for West Nile Virus Circulation Risk, Camargue, Southern France. International Journal of Environmental Research and Public Health. 2014; 11(8):7740-7754. https://doi.org/10.3390/ijerph110807740
Chicago/Turabian StylePradier, Sophie, Alain Sandoz, Mathilde C. Paul, Gaëtan Lefebvre, Annelise Tran, Josiane Maingault, Sylvie Lecollinet, and Agnès Leblond. 2014. "Importance of Wetlands Management for West Nile Virus Circulation Risk, Camargue, Southern France" International Journal of Environmental Research and Public Health 11, no. 8: 7740-7754. https://doi.org/10.3390/ijerph110807740
APA StylePradier, S., Sandoz, A., Paul, M. C., Lefebvre, G., Tran, A., Maingault, J., Lecollinet, S., & Leblond, A. (2014). Importance of Wetlands Management for West Nile Virus Circulation Risk, Camargue, Southern France. International Journal of Environmental Research and Public Health, 11(8), 7740-7754. https://doi.org/10.3390/ijerph110807740