Full-Scale Experimental Study of Groundwater Softening in a Circulating Pellet Fluidized Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
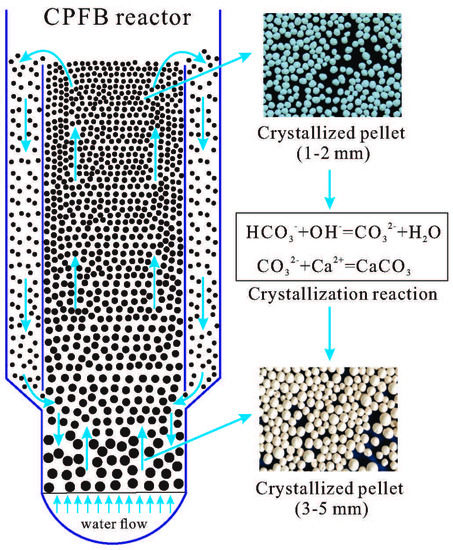
2.2. Full-Scale Experimental System
2.3. Experiment Process Description
2.4. Analysis Methods
3. Results and Discussion
3.1. Experimental Study on NaOH and HCl Dosage Optimization
3.2. Experimental Study on Pellet Distribution and Hardness Removal Characteristics at Different Heights of CPFB Reactor
3.3. Experimental Study on Pellet Growth
3.4. Experimental Study on the Relationship between Pressure and Bed Height Variation and Pellet Discharge
3.5. Costs
4. Conclusions
- In the CPFB reactor, the removal rate of Ca2+ and TH can reach 90% and 60%, respectively, and the effluent pH can be controlled between 9.5–9.8. The turbidity of the effluent and the turbidity after boiling are stable at less than 1.0 NTU. The unit water treatment cost is less than €0.064 per m3. The CPFB reactor has advantages in terms of the softening effect and cost.
- The unique structure of the CPFB reactor improves the crystallization efficiency, increases the utilization of the seed material, and extends the discharge time of mature pellets. The size of the discharged pellets can reach 3–5 mm, and the height of the CPFB reactor is reduced from between 5–6 m to 4 m.
Author Contributions
Funding
Conflicts of Interest
References
- Hofman, J.; Kramer, O.; Hoek, J.P.V.D.; Nederlof, M.; Groenendijk, M. Twenty years of experience with central softening in The Netherlands: Water quality, environmental benefits, and costs. In Proceedings of the International Symposium on Health Aspects of Calcium and Magnesium in Drinking Water, Baltimore, MD, USA, 27–28 April 2006. [Google Scholar]
- Kramer, O.J.I.; Jobse, M.A.; Baars, E.T.; van der Helm, A.W.C.; Colin, M.G.; Kors, L.J.; van Vugt, W.H. Model-based prediction of fluid bed state in full-scale drinking water pellet softening reactors. In Proceedings of the 2nd IWA New Developments in IT & Water Conference, Hague, The Netherlands, 8–10 February 2015. [Google Scholar]
- He, C.; Gross, M.; Westerhoff, P.; Fox, P.; Li, K. Comparing Conventional and Pelletized Lime Softening Concentrate Chemical Stabilization; Water Research Foundation: Denver, CO, USA; Phoenix Water Services Department: Phoenix, AZ, USA, 2011. [Google Scholar]
- Pratomo, U.; Anggraeni, A.; Lubis, R.A.; Pramudya, A.; Farida, I.N. Study of softening hard water using Pistacia vera shell as adsorbent for calcium and magnesium removal. Procedia Chem. 2015, 16, 400–406. [Google Scholar] [CrossRef]
- Van Schagen, K.M.; Babuška, R.; Rietveld, L.C.; Veersma, A.M.J. Model-based dosing control of a pellet softening reactor. IFAC Proc. Vol. 2009, 42, 267–272. [Google Scholar] [CrossRef]
- Graveland, A.; van Dijk, J.C.; de Moel, P.J.; Oomen, J.H.C.M. Developments in water softening by means of pellet reactors. J.-Am. Water Works Assoc. 1983, 75, 619–625. [Google Scholar] [CrossRef]
- Van der Veen, C.; Graveland, A. Central softening by crystallization in a fluidized-bed process. J.-Am. Water Works Assoc. 1988, 80, 51–58. [Google Scholar] [CrossRef]
- Schetters, M.J.A.; van der Hoek, J.P.; Kramer, O.J.; Kors, L.J.; Palmen, L.J.; Hofs, B.; Koppers, H. Circular economy in drinking water treatment: Reuse of ground pellets as seeding material in the pellet softening process. Water Sci. Technol. 2015, 71, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Schetters, M.J.A. Grinded Dutch Calcite as Seeding Material in the Pellet Softening Process. Master’s Thesis, TU Delft, Delft, The Netherlands, 2013. [Google Scholar]
- Schagen, K.M.V.; Rietveld, L.C.; Babuška, R. Dynamic modelling for optimisation of pellet softening. J. Water Supply Res. Technol. 2008, 57, 45–56. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, R.; An, D.; Cheng, Y.; Tan, H. Water softening by induced crystallization in fluidized bed. J. Environ. Sci. (Engl. Ed.) 2016, 50, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.Z.; Huang, T.L.; Wen, G.; Yang, S. Modelling particle growth of calcium carbonate in a pilot-scale pellet fluidized bed reactor. Water Sci. Technol. Water Supply 2017, 17, 643–651. [Google Scholar] [CrossRef]
- Rietveld, L.C.; Schagen, K.M.V.; Kramer, O.J.I. Optimal operation of the pellet softening process. In Proceedings of the AWWA Workshop, Austin, TX, USA, 29–31 January 2006. [Google Scholar]
- Hammes, F.; Boon, N.; Vital, M.; Ross, P.; Magic-Knezev, A.; Dignum, M. Bacterial colonization of pellet softening reactors used during drinking water treatment. Appl. Environ. Microbiol. 2011, 77, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhou, K.G.; Yang, Y.C.; Du, H. Growth kinetics of calcium fluoride at high supersaturation in a fluidized bed reactor. Environ. Technol. 2014, 35, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.Y. Crystal growth kinetics of two-step growth process in liquid fluidized-bed crystallizers. J. Cryst. Growth 1999, 206, 109–118. [Google Scholar] [CrossRef]
- Su, C.C.; Dulfo, L.D.; Dalida, M.L.P.; Lu, M.C. Magnesium phosphate crystallization in a fluidized-bed reactor: Effects of pH, Mg:P molar ratio and seed. Sep. Purif. Technol. 2014, 125, 90–96. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Shafiee, F.; Naddafi, K. Feasibility study of crystallization process for water softening in a pellet reactor. Int. J. Environ. Sci. Technol. 2005, 1, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Garea, A.; Aldaco, R.; Irabien, A. Improvement of calcium fluoride crystallization by means of the reduction of fines formation. Chem. Eng. J. 2009, 154, 231–235. [Google Scholar] [CrossRef]
- Van Schagen, K.; Rietveld, L.; Babuška, R.; Baars, E. Control of the fluidised bed in the pellet softening process. Chem. Eng. Sci. 2008, 63, 1390–1400. [Google Scholar] [CrossRef]
- Aldaco, R.; Garea, A.; Irabien, A. Particle growth kinetics of calcium fluoride in a fluidized bed reactor. Chem. Eng. Sci. 2007, 62, 2958–2966. [Google Scholar] [CrossRef]
- Tai, C.Y.; Hsu, H.P. Crystal growth kinetics of calcite and its comparison with readily soluble salts. Powder Technol. 2001, 121, 60–67. [Google Scholar] [CrossRef]
- Aldaco, R.; Garea, A.; Irabien, A. Modeling of particle growth: Application to water treatment in a fluidized bed reactor. Chem. Eng. J. 2007, 134, 66–71. [Google Scholar] [CrossRef]








| No. | Water Quality | Value |
|---|---|---|
| 1 | pH | 7.6–7.7 |
| 2 | Temperature/°C | 18–21 |
| 3 | Turbidity of raw water/NTU | <1 |
| 4 | Turbidity after boiling/NTU | 90–100 |
| 5 | Total alkalinity (CaCO3)/(mg/L) | 262 |
| 6 | Bicarbonate alkalinity (CaCO3)/(mg/L) | 262 |
| 7 | Total hardness (TH) (CaCO3)/(mg/L) | 286 |
| 8 | Ca2+ (mg/L) | 75 |
| 9 | Mg2+ (mg/L) | 24 |
| No. | Equipment Name | Parameter | Remarks |
|---|---|---|---|
| 1 | Pipeline pump | H = 0–25 m, Q = 200 m3/h, P = 15 kW | Frequency conversion pump |
| 2 | CPFB reactor | D = 1.6 m, H = 4.0 m | Stainless steel |
| 3 | Acid bucket | V = 12 m3 | Polyethylene, design for 7 days |
| 4 | NaOH bucket | V = 12 m3 | Polyethylene, design for 10 days |
| 5 | Pellet storage box | V = 15 m3 | Carbon steel |
| No. | Parameter Name | Value |
|---|---|---|
| 1 | Superficial velocity/m/h | 60–100 |
| 2 | NaOH dosage/mg/L | 38–150 |
| 3 | HCl dosage/mg/L | 16–80 |
| 4 | Pellet discharge/kgCaCO3/day | 300–400 |
| 5 | Garnet dosage/kg/time/day | 25–50 |
| 6 | pH before acidification | 9.5–9.9 |
| 7 | pH after acidification | 7.0–8.0 |
| No. | Cost Composition | Euro | Percentage (%) |
|---|---|---|---|
| 1 | NaOH | 69,228 | 65 |
| 2 | HCl | 19,230 | 18 |
| 3 | Garnet | 1154 | 1 |
| 4 | Energy | 13,461 | 12 |
| 5 | Labor | 3846 | 4 |
| 6 | Total cost | 106,919 | 100 |
| 7 | Unit cost (€ per m3) | 0.058 | / |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Huang, T.; Zhi, A.; Tang, Z. Full-Scale Experimental Study of Groundwater Softening in a Circulating Pellet Fluidized Reactor. Int. J. Environ. Res. Public Health 2018, 15, 1592. https://doi.org/10.3390/ijerph15081592
Hu R, Huang T, Zhi A, Tang Z. Full-Scale Experimental Study of Groundwater Softening in a Circulating Pellet Fluidized Reactor. International Journal of Environmental Research and Public Health. 2018; 15(8):1592. https://doi.org/10.3390/ijerph15081592
Chicago/Turabian StyleHu, Ruizhu, Tinglin Huang, Aofan Zhi, and Zhangcheng Tang. 2018. "Full-Scale Experimental Study of Groundwater Softening in a Circulating Pellet Fluidized Reactor" International Journal of Environmental Research and Public Health 15, no. 8: 1592. https://doi.org/10.3390/ijerph15081592
APA StyleHu, R., Huang, T., Zhi, A., & Tang, Z. (2018). Full-Scale Experimental Study of Groundwater Softening in a Circulating Pellet Fluidized Reactor. International Journal of Environmental Research and Public Health, 15(8), 1592. https://doi.org/10.3390/ijerph15081592