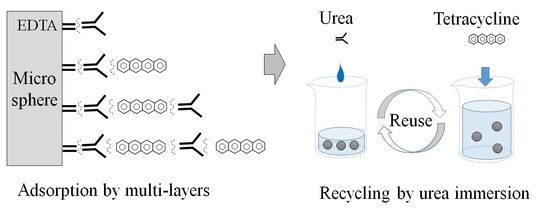
Simple Urea Immersion Enhanced Removal of Tetracycline from Water by Polystyrene Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microsphere Modification
2.2. Surface Characterization
2.3. Chemicals and Analysis
2.4. Adsorption Experiments
2.5. Recycling Adsorption by Urea Immersion
2.6. Data Interpretation
3. Results and Discussion
3.1. Solid Surface Characterization
3.1.1. Surface Characterization
3.1.2. FT-IR Analysis
3.1.3. XPS Analysis
3.2. Adsorption Isotherms and Kinetics
3.2.1. Isothermal Modeling
3.2.2. Kinetics Modeling
3.2.3. Adsorption Performance
3.3. Recycling Adsorption and Desorption
3.3.1. Adsorption by Cycle Urea Immersion
3.3.2. Desorption Performance
3.4. Adsorption Mechanisms
3.4.1. FT-IR Analysis after Adsorption
3.4.2. Role of Urea for Multilayer Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulkarni, P.; Olson, N.; Raspanti, G.; Rosenberg Goldstein, R.; Gibbs, S.; Sapkota, A.; Sapkota, A. Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water. Int. J. Environ. Res. Public Health 2017, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Hladicz, A.; Kittinger, C.; Zarfel, G. Tigecycline Resistant Klebsiella pneumoniae Isolated from Austrian River Water. Int. J. Environ. Res. Public Health 2017, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Yang, Q.; Sun, L.; Yang, X.; Zhou, M.; Deng, R.; Bi, L. Plant Growth, Antibiotic Uptake, and Prevalence of Antibiotic Resistance in an Endophytic System of Pakchoi under Antibiotic Exposure. Int. J. Environ. Res. Public Health 2017, 14, 1336. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.; Queirós, D.B.; Reis, A.C.; Nunes, O.C.; Borges, M.T.; Boaventura, R.A.; Vilar, V.J. Process enhancement at near neutral pH of a homogeneous photo-Fenton reaction using ferricarboxylate complexes: Application to oxytetracycline degradation. Chem. Eng. J. 2014, 253, 217–228. [Google Scholar] [CrossRef] [Green Version]
- López Peñalver, J.J.; Sánchez Polo, M.; Gómez Pacheco, C.V.; Rivera Utrilla, J. Photodegradation of tetracyclines in aqueous solution by using UV and UV/H2O2 oxidation processes. J. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Qi, Z.; Diao, M.; Gao, M.; Xing, S.; Wang, S.; Zhao, X. Sorption and biodegradation of tetracycline by nitrifying granules and the toxicity of tetracycline on granules. J. Hazard. Mater. 2011, 191, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Sirés, I.; Oturan, N.; Özcan, A.; Oturan, M.A. Electrochemical treatment of the antibiotic sulfachloropyridazine: Kinetics, reaction pathways, and toxicity evolution. Environ. Sci. Technol. 2012, 46, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Huang, L.; Li, L.; Gielen, G.; Wang, H.; Zhang, Y. Degradation of Tetracyclines in Pig Manure by Composting with Rice Straw. Int. J. Environ. Res. Public Health 2016, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Khamparia, S.; Jaspal, D.K. Adsorption in combination with ozonation for the treatment of textile waste water: A critical review. Front. Environ. Sci. Eng. 2017, 11, 8. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; de Yuso, A.M.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wu, L.; Lan, H.; Qu, J. Preparation of amino-Fe (III) functionalized mesoporous silica for synergistic adsorption of tetracycline and copper. Chemosphere 2015, 138, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Turku, I.; Sainio, T.; Paatero, E. Thermodynamics of tetracycline adsorption on silica. Environ. Chem. Lett. 2007, 5, 225–228. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, F.; Lu, Y.; Xue, X.; Li, N. Adsorption interaction of tetracyclines with porous synthetic resins. Ind. Eng. Chem. Res. 2011, 50, 13892–13898. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, M.C.; Shuang, C.D.; Li, Z.Q.; Li, A.M. Preparation of a novel magnetic powder resin for the rapid removal of tetracycline in the aquatic environment. Chin. Chem. Lett. 2012, 23, 745–748. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Ye, Z.; Wu, P.; Wei, N.; Wu, X.; Li, H. Preparation of metal ions impregnated polystyrene resins for adsorption of antibiotics contaminants in aquatic environment. J. Appl. Polym. Sci. 2015, 132, 41803. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Q.; Li, A.; Shuang, C.; Shi, Q.; Zhang, M. Preparation of a novel magnetic microporous adsorbent and its adsorption behavior of p-nitrophenol and chlorotetracycline. Hazard. Mater. 2014, 266, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Zhu, W.; Yan, B.; Lin, Y.; Xun, S.; Ji, H.; Wu, X.; Li, H.; Han, C. Macroporous polystyrene resins as adsorbents for the removal of tetracycline antibiotics from an aquatic environment. J. Appl. Polym. Sci. 2014, 131, 40561. [Google Scholar] [CrossRef]
- Hao, R.; Xiao, X.; Zuo, X.; Nan, J.; Zhang, W. Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres. J. Hazard. Mater. 2012, 209, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Liu, H. Insight into the adsorption of tetracycline onto amino and amino-Fe3+ gunctionalized mesoporous silica: Effect of functionalized groups. J. Environ. Sci. 2018, 65, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Ma, Y.; Chang, X.; Fan, S. Removal and removing mechanism of tetracycline residue from aqueous solution by using Cu-13X. Chem. Eng. J. 2015, 273, 247–253. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Wang, L.; Zhang, Y.; Ma, X.; Ye, Z. Preparation and adsorption performance of a novel bipolar PS-EDTA resin in aqueous phase. J. Hazard. Mater. 2010, 180, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Li, Y.; Ye, Z.; He, A. Efficient Removal of Cd2+ from Aqueous Solutions by Adsorption on PS-EDTA Resins: Equilibrium, Isotherms, and Kinetic Studies. J. Environ. Eng. ASCE 2012, 138, 940–948. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Li, Y.; Zhang, Y.; Ma, X.; Ye, Z. Study on adsorption mechanism of Pb(II) and Cu(II) in aqueous solution using PS-EDTA resin. Chem. Eng. J. 2010, 163, 364–372. [Google Scholar] [CrossRef]
- Li, B.; Ma, J.; Zhou, L.; Qiu, Y. Magnetic microsphere to remove tetracycline from water: Adsorption, H2O2 oxidation and regeneration. Chem. Eng. J. 2017, 330, 191–201. [Google Scholar] [CrossRef]
- He, J.; Dai, J.; Xie, A.; Tian, S.; Chang, Z.; Yan, Y.; Huo, P. Preparation of macroscopic spherical porous carbons@carboxymethylcellulose sodium gel beads and application for removal of tetracycline. RSC Adv. 2016, 6, 84536–84546. [Google Scholar] [CrossRef]
- Chen, L.; Lei, S.; Wang, M.; Yang, J.; Ge, X. Fabrication of macroporous polystyrene/graphene oxide composite monolith and its adsorption property for tetracycline. Chin. Chem. Lett. 2016, 27, 511–517. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Y.; Zhong, L.; Cheng, X. Removal of tetracycline from aqueous solution by a Fe3O4 incorporated PAN electrospun nanofiber mat. J. Environ. Sci. 2015, 28, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.K.; Snisarenko, O.; Lee, H.S.; Shin, E.W. Adsorption of tetracycline on La-impregnated MCM-41 materials. Environ. Technol. 2010, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shi, L.; Shen, H.; Chen, X.; Xie, K. Equilibrium, isotherm, kinetic and thermodynamic studies for removal of tetracycline antibiotics by adsorption onto hazelnut shell derived activated carbons from aqueous media. RSC Adv. 2016, 6, 109983–109991. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, M.; Wang, W.; Zhou, Q.; Liu, F. Efficient and synergistic removal of tetracycline and Cu(II) using novel magnetic multi-amine resins. Sci. Rep. 2018, 8, 4762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, B.; An, Q.; Xiao, Z.; Zhai, S. Hydrophilic, hollow Fe3O4@PDA spheres with a storage cavity for efficient removal of polycyclic structured tetracycline. New J. Chem. 2017, 41, 1235–1244. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Li, A.; Shuang, C.; Zhang, M.; Liu, X.; Wu, L. Preparation of a novel anion exchange group modified hyper-crosslinked resin for the effective adsorption of both tetracycline and humic acid. Front. Environ. Sci. Eng. 2013, 7, 412–419. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Wang, H.; Tan, X.; Yang, Y.; Liu, Y. Efficient Removal of Tetracycline from Aqueous Media with a Fe3O4 Nanoparticles@graphene Oxide Nanosheets Assembly. Int. J. Environ. Res. Public Health 2017, 14, 1495. [Google Scholar] [CrossRef] [PubMed]
- Soori, M.M.; Ghahramani, E.; Kazemian, H.; Al-Musawi, T.J.; Zarrabi, M. Intercalation of tetracycline in nano sheet layered double hydroxide: An insight into UV/VIS spectra analysis. J. Taiwan Inst. Chem. Eng. 2016, 63, 271–285. [Google Scholar] [CrossRef]
- Fu, D.; Huang, Y.; Zhang, X.; Kurniawan, T.A.; Ouyang, T. Uncovering potentials of integrated TiO2(B) nanosheets and H2O2 for removal of tetracycline from aqueous solution. J. Mol. Liq. 2017, 248, 112–120. [Google Scholar] [CrossRef]
- Borghi, A.A.; Palma, M.S.A. Tetracycline: Production, waste treatment and environmental impact assessment. Braz. J. Pharm. Sci. 2014, 50, 25–40. [Google Scholar] [CrossRef]









| Model Name | Equation | Lineweaver–Burk Equation | Coefficients |
|---|---|---|---|
| Langmuir | qe = qm·Ce/(KL + Ce) | Ce/qe = Ce/qm + KL/qm | KL, qm |
| Freundlich | qe = Kf·Ce1/n | lnqe = ln Kf + 1/n·lnCe | Kf, n |
| Tempkin | qe = RT/b·ln(a·Ce) | qe = RT/b·lna + RT/b·lnCe | a, RT/b |
| First-order | dqt/dt = K1·(qe − qt) | ln(qe − qt) = lnqe − K1·t | K1 |
| Second-order | dqt/dt = K2·(qe − qt)2 | 1/t = (K2·qe2) (1/qt − 1/qe) | K2 |
| Weber–Morris | qt = qe·Kw·t1/2 | qt/qe = Kw·t1/2 | Kw |
| Adsorbent | Adsorbate | Langmuir | Freundlich | Tempkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm | KL | R2 | Kf | n | R2 | a | RT/b | R2 | ||
| PSM | TC | 290 | 180 | 0.960 | 15 | 2.4 | 0.996 | 0.06 | 59 | 0.952 |
| DC | 330 | 290 | 0.923 | 9.4 | 2.1 | 0.983 | 0.14 | 83 | 0.919 | |
| UPSM | TC | 460 | 120 | 0.966 | 25 | 2.3 | 0.955 | 0.06 | 57 | 0.871 |
| DC | 430 | 56 | 0.985 | 35 | 2.5 | 0.940 | 0.47 | 67 | 0.830 | |
| Target | First Rate Constant K1 | R2 | Second Rate Constant K2qe2 | R2 | Weber–Morris Constant Kw | R2 |
|---|---|---|---|---|---|---|
| /h | mg/g/h | /h1/2 | ||||
| TC | 0.41 | 0.942 | 1.8 | 0.939 | 0.64 | 0.963 |
| DC | 0.33 | 0.929 | 2.1 | 0.933 | 0.63 | 0.962 |
| New Adsorbent | qm, mg/g | Reference |
|---|---|---|
| Magnetic multiamine resins | 117 | [35] |
| Magnetic polystyrene resins | 166 | [29] |
| Magnetic polydopamine resins | 152 | [36] |
| Polystyrene microsphere/graphene oxide | 198 | [31] |
| Polymer resins/anion exchange group | 355 | [37] |
| Magnetic microsphere/graphene oxide nanosheet | 714 | [38] |
| Nanosheet-layered double hydroxide | 98 | [39] |
| TiO2 nanosheets | 213 | [40] |
| Magnetic polyacrylonitrile nanofiber mat | 315 | [32] |
| Amino-ferrous functionalized silica | 188 | [24] |
| La-impregnated silicates | 303 | [33] |
| Activated carbons from hazelnut shell | 303 | [34] |
| Urea functionalized polystyrene resins | 460 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Li, B.; Zhou, L.; Zhu, Y.; Li, J.; Qiu, Y. Simple Urea Immersion Enhanced Removal of Tetracycline from Water by Polystyrene Microspheres. Int. J. Environ. Res. Public Health 2018, 15, 1524. https://doi.org/10.3390/ijerph15071524
Ma J, Li B, Zhou L, Zhu Y, Li J, Qiu Y. Simple Urea Immersion Enhanced Removal of Tetracycline from Water by Polystyrene Microspheres. International Journal of Environmental Research and Public Health. 2018; 15(7):1524. https://doi.org/10.3390/ijerph15071524
Chicago/Turabian StyleMa, Junjun, Bing Li, Lincheng Zhou, Yin Zhu, Ji Li, and Yong Qiu. 2018. "Simple Urea Immersion Enhanced Removal of Tetracycline from Water by Polystyrene Microspheres" International Journal of Environmental Research and Public Health 15, no. 7: 1524. https://doi.org/10.3390/ijerph15071524
APA StyleMa, J., Li, B., Zhou, L., Zhu, Y., Li, J., & Qiu, Y. (2018). Simple Urea Immersion Enhanced Removal of Tetracycline from Water by Polystyrene Microspheres. International Journal of Environmental Research and Public Health, 15(7), 1524. https://doi.org/10.3390/ijerph15071524