Determination of Triglycidyl Isocyanurate in Workplace Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Material and Reagents
3. Methodology
3.1. Sample Preparation
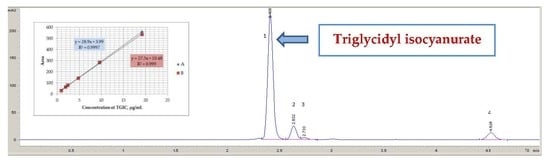
3.2. Chromatographic Conditions
3.3. Recovery Rate Studies
3.4. Calibration and Precision
3.5. Method Validation
- b – slope coefficient of the calibration curve;
- so – standard deviation of results obtained for a series of blank samples.
- Vp – is the precision of the sampling device (Vp = ± 5%);
- Vz – mean precision of three levels of ranges, which has been calculated using the formula:
- nj is the number of duplicated samples (nj = 8); and
- Vi is the coefficient of variation for a given level of concentration.
4. Results and Discussion
4.1. Tests of the Efficiency of TGIC Recovery from Filters
4.2. Sampling
4.3. Recovery Rate Studies
4.4. Calibration and Precision
4.5. Sample Storage
4.6. Validation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jelonek, A. Weatherproof polyester powder paints. Lakiernictwo Przemysłowe 2004, 6, 1–5. (In Polish) [Google Scholar]
- Willcocks, D.; Onyon, L.; Jenkins, C.; Biver, B. Triglycidyl isocyanurate. In Concise International Chemical Assessment Document 8; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Mathias, C.G.T. Allergic contact dermatitis from triglycidyl isocyanurate in polyester paint pigments. Contact Dermat. 1988, 19, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Ogasawara, M.; Asagami, C. Occupational contact allergy to triglycidyl isocyanurate (TGIC, Tepic®). Contact Dermat. 1988, 19, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.S.; Lawrence, C.M. Occupational contact dermatitis from triglycidyl isocyanurate in a powder paint factory. Contact Dermat. 1992, 26, 59. [Google Scholar] [CrossRef] [PubMed]
- Jolanki, R.; Kanerva, L.; Estlander, T.; Tarvainen, K. Concomitant sensitization to triglycidyl isocyanurate. diaminodiphenylmethane and 2-hydroxyethyl methacrylate from silk-screen printing coatings in the manufacture of circuit boards. Contact Dermat. 1994, 30, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Piirilä, P.; Estlander, T.; Keskinen, H.; Jolanki, R.; Laakkonen, A.; Pfäffli, P.; Tupasela, O.; Tuppurainen, M.; Nordman, H. Occupational asthma caused by triglycidyl isocyanurate (TGIC). Clin. Exp. Allergy 1997, 27, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Wigger-Alberti, W.; Hofmann, M.; Elsner, P. Contact dermatitis caused by triglycidyl isocyanurate. Am. J. Contact Dermat. 1997, 8, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Craven, N.M.; Bhushan, M.; Beck, M.H. Sensitization to triglycidyl isocyanurate, epoxy resins and acrylates in a developmental chemist. Contact Dermat. 1999, 40, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Erikstam, U.; Bruze, M.; Goossens, A. Degradation of triglycidyl isocyanurate as a cause of false-negative patch test reaction. Contact Dermat. 2001, 44, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Aalto-Korte, K.; Suuronen, K. Occupational contact allergy to components of polyester resin systems. Contact Dermat. 2016, 75, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Anees, W.; Moore, V.C.; Croft, J.S.; Robertson, A.S.; Burge, P.S. Occupational asthma caused by heated triglycidyl isocyanurate. Occup. Med. 2011, 61, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU). 2018/669 of 16 April 2018 amending, for the purposes of its adaptation to technical and scientific progress. Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. 4.5.2018 (L 115/1). Available online: http://data.europa.eu/eli/reg/2018/669/oj (accessed on 22 October 2019).
- GESTIS International Limit Values Database; BG Institute for Occupational Safety and Health: Sankt Augustin, Germany; Available online: https://limitvalue.ifa.dguv.de/ (accessed on 16 May 2019).
- Lee, D. 1.3.5-Triglycidyl Isocyanurate. In Method OSHA no PV 2055. Carcinogen and Pesticide Branch; OSHA Analytical Laboratory: Salt Lake City, UT, USA, 1988. [Google Scholar]
- Health and Safety Executive (HSE). Triglycidyl Isocyanurate (and Coating Powders Containing Triglycidyl Isocyanurate) in Air; Method MDHS 85/2; HSE (Health and Safety Executive): Sudbury, UK, 2015.
- Gagné, S.; Carrier, M.; Aubin, S. Determination of triglycidyl isocyanurate from air samples by ultra-performance liquid chromatography coupled with coordination ion spray mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 913–918. [Google Scholar] [CrossRef] [PubMed]
- EN 482:2012+A1:2015. Workplace Exposure—General Requirements for the Performance of Procedures for the Measurement of Chemical Agents; European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- PN-Z-04008-7:2002. Air Purity Protection—Sampling Methods—Principles of Air Sampling in Work Place and Interpretation of Results; Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish) [Google Scholar]
- Liden, G.; Melin, B.; Lidblom, A.; Lindberg, K.; Noren, J.O. Personal Sampling in Parallel with Open-Face Filter Cassettes and IOM Samplers for Inhalable Dust—Implications for Occupational Exposure Limits. Appl. Occup. Environ. Hyg. 2000, 3, 263–276. [Google Scholar] [CrossRef] [PubMed]
- EN 689:2018+AC:2019. Workplace Exposure—Measurement of Exposure by Inhalation to Chemical Agents.—Strategy for Testing Compliance with Occupational Exposure Limit Values; European Committee for Standardization: Brussels, Belgium, 2019. [Google Scholar]


| Hazard Class and Category Codes | Hazard Statements Codes |
|---|---|
| Muta. 1B | H340 May cause genetic defects |
| Acute Tox. 3 | H331 Toxic if inhaled |
| Acute Tox. 3 | H301 Toxic if swallowed |
| STOT RE 2 | H373 May cause damage to organs through prolonged or repeated exposure |
| Eye Dam. 1 | H318 Causes serious eye damage |
| Skin Sens. 1 | H317 May cause an allergic skin reaction |
| Aquatic Chronic 3 | H412 Harmful to aquatic life with long-lasting effects |
| Country | Limit Value—Eight Hours [mg/m3] |
|---|---|
| Australia | 0.08 |
| Belgium | 0.05 |
| Canada—Ontario | 0.05 |
| Finland | 0.1 |
| Ireland | 0.05 |
| New Zealand | 0.08 |
| Spain | 0.05 |
| United Kingdom | 0.1 |
| Type of Filter | Average Area of TGIC Peaks from Recovered Solutions | Average Area of TGIC Peaks from Comparative Solutions | Recovery Rate | Average Recovery Rate |
|---|---|---|---|---|
| PTFE | 29.6 | 71.75 | 0.41 | 0.37 |
| 22.8 | 0.32 | |||
| 26.8 | 0.37 | |||
| FIPRO | 70.7 | 71.75 | 0.99 | 0.98 |
| 71.3 | 0.99 | |||
| 69.9 | 0.97 | |||
| GF/A | 70.2 | 71.75 | 1.10 | 0.98 |
| 70.5 | 1.09 | |||
| 71.2 | 1.10 |
| Mass of TGIC on the Filter [µg] | Air Flow Rate [L/min] | Area of the TGIC Peaks in Solution: | |
|---|---|---|---|
| After Recovery from the Filter | Comparative | ||
| 28.8 | 2 | 861.9 | 874.5 |
| 880.1 | |||
| 869.2 | |||
| Concentration of TGIC Solution [µg/mL] | Average Area of Peaks from Recovered Solutions | Average Area of Peaks from Comparative Solutions | Relative Standard Deviation [%] | Average Recovery Rate |
|---|---|---|---|---|
| 0.96 | 28.7 | 28.8 | 4.43 | 0.99 |
| 9.6 | 273.1 | 265.1 | 3.34 | 1.03 |
| 19.2 | 509.0 | 505.0 | 1.35 | 1.01 |
| Parameter | Measurement Series | ||
|---|---|---|---|
| I | II | III | |
| Concentration of the solution [μg/mL] | 1.92 | 9.6 | 19.2 |
| Average value of peak area | 60.09 | 301.74 | 567.23 |
| Standard deviation | 1.57 | 5.77 | 3.75 |
| Coefficient of variation [%] | 2.61 | 1.91 | 0.66 |
| Filter No. | Storage Place | Storage Time [Number of Days] | Average Peak Area of the TGIC | Two-Filter Average | Standard Deviation |
|---|---|---|---|---|---|
| 1 | desiccator | 1 | 293.30 | 293.35 | 0.1 |
| 2 | 293.40 | ||||
| 1 | refrigerator | 1 | 279.00 | 279.85 | 1.2 |
| 2 | 280.70 | ||||
| 1 | desiccator | 5 | 273.45 | 272.10 | 1.9 |
| 2 | 270.75 | ||||
| 1 | refrigerator | 5 | 282.25 | 278.10 | 5.9 |
| 2 | 273.95 | ||||
| 1 | desiccator | 7 | 300.60 | 301.38 | 1.1 |
| 2 | 302.15 | ||||
| 1 | refrigerator | 7 | 302.65 | 300.53 | 3.0 |
| 2 | 298.40 |
| Parameter | Value |
|---|---|
| Measurement range | 0.002–0.04 mg/m3 |
| Sampled air volume | 720 L |
| Range of calibration curve | 0.96–19.2 µg/mL |
| Limit of detection (LOD) | 11.2 ng/mL (23.3 ng/m3) |
| Limit of quantitation (LOQ) | 33.6 ng/mL (70 ng/m3) |
| Overall precision of examination | 5.35% |
| Relative total uncertainty | 12% |
| Relative expanded uncertainty | 24% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeżewska, A.; Kowalska, J. Determination of Triglycidyl Isocyanurate in Workplace Air. Int. J. Environ. Res. Public Health 2019, 16, 4455. https://doi.org/10.3390/ijerph16224455
Jeżewska A, Kowalska J. Determination of Triglycidyl Isocyanurate in Workplace Air. International Journal of Environmental Research and Public Health. 2019; 16(22):4455. https://doi.org/10.3390/ijerph16224455
Chicago/Turabian StyleJeżewska, Anna, and Joanna Kowalska. 2019. "Determination of Triglycidyl Isocyanurate in Workplace Air" International Journal of Environmental Research and Public Health 16, no. 22: 4455. https://doi.org/10.3390/ijerph16224455
APA StyleJeżewska, A., & Kowalska, J. (2019). Determination of Triglycidyl Isocyanurate in Workplace Air. International Journal of Environmental Research and Public Health, 16(22), 4455. https://doi.org/10.3390/ijerph16224455