Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México
Abstract
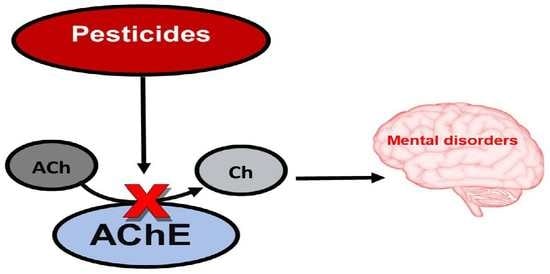
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participant Selection
2.2. Ethical Statement
2.3. Data Collection
2.4. Sample Preparation
2.5. Determination of AChE Activity
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, P. Anticholinesterases. In Goodman and Gilman’s Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Ed.; McGraw Hill Companies: New York, NY, USA, 2009; pp. 239–254. [Google Scholar]
- Taylor, P. Anticholinesterase Agents. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Ed.; McGraw Hill Companies: New York, NY, USA, 2017; pp. 163–176. [Google Scholar]
- Hagstrom, D.; Zhang, S.; Ho, A.; Tsai, E.S.; Radić, Z.; Jahromi, A.; Kaj, K.J. Planarian cholinesterase: Molecular and functional characterization of an evolutionarily ancient enzyme to study organophosphorus pesticide toxicity. Arch. Toxicol. 2018, 92, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Aaron, C.K. Organophosphate and carbamate poisoning. Emerg. Med. Clin. 2015, 33, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J. Research|Children’s Health Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The CHARGE Study. Environ. Health Perspect. 2014, 122, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, D.; Hirokawa, H.; Zhang, L.; Radic, Z.; Taylor, P.; Maria, E.; Taylor, P. Planarian cholinesterase: In vitro characterization of an evolutionarily ancient enzyme to study organophosphorus pesticide toxicity and reactivation. Arch. Toxicol. 2016, 91, 2834–2847. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.T.; Boris, A.L.; Barr, D.B.; Steenland, K.; Levy, K.; Ryan, P.B.; Iglesias, V.; Alvarado, S.; Concha, C.; Rojas, E.; et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. Neurotoxicology 2013, 39, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altinyazar, V.; Sirin, F.B.; Sutcu, R.; Eren, I.; Omurlu, I.K. The Red Blood Cell Acetylcholinesterase Levels of Depressive Patients with Suicidal Behavior in an Agricultural Area. Indian J. Clin. Biochem. 2016, 31, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.M.; Mcmanus, I.C.; Harrison, V.; Mason, O. Eurobehavioral problems following low-level exposure to organophosphate pesticides: A systematic and meta-analytic review. Crit. Rev. Toxicol. 2013, 43, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.T.; Dinis-Oliveira, R.J.; de Lourdes Bastos, M.; Tsatsakis, A.M.; Duarte, J.A.; Carvalho, F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases a mechanistic approach. Toxicol. Lett. 2014, 230, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Bellinger, D.C.; Wright, R.O.; Marc, G. Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides. Pediatrics 2010, 125, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Koifman, S. Pesticides, depression and suicide: A systematic review of the epidemiological evidence. Int. J. Hyg. Environ. Health 2013, 216, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Agenda Técnica Agrícola, 2nd ed.; INIFAP; SAGARPA: México City, México, 2015. Available online: https://extensionismo.sagarpa.gob.mx/web2/documentos/agenda_tecnica/F02_BajaCalifornia.pdf (accessed on 19 February 2019).
- Gobierno del Estado de Baja California. Plan Estatal de Desarrollo 2014–2019. Available online: http://www.bajacalifornia.gob.mx/portal/gobierno/ped/ped.jsp (accessed on 19 February 2019).
- Ayala, A.V.; Schwentesius, R.; Carrera, B. Hortalizas en México: Competitividad frente a EE.UU. y oportunidades de desarrollo. J. Glob. Compet. Gov. 2012, 6, 70–88. [Google Scholar]
- Hernández, A.; Hansen, A.M. Uso de plaguicidas en dos zonas agrícolas de México y evaluación de la contaminación de agua y sedimentos. Rev. Int. Contam. Ambient. 2011, 27, 115–127. [Google Scholar]
- Worek, F.; Mast, U.; Kiderlen, D.; Diepold, C.; Eyer, P. Improved determination of acetylcholinesterase activity in human whole blood. Clin. Chim. Acta 1999, 288, 73–90. [Google Scholar] [CrossRef]
- Lecrubier, Y.; Sheehan, D.V.; Weiller, E.; Amorim, P.; Bonora, I.; Sheehan, K.H.; Dunbar, G.C. Original article The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur. Psychiatry 1997, 12, 224–231. [Google Scholar] [CrossRef]
- Pinninti, N.R.; Madison, H.; Musser, E.; Rissmiller, D. MINI International Neuropsychiatric Schedule: Clinical utility and patient acceptance. Eur. Psychiatry 2003, 18, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Mordal, J.; Gundersen, Ø.; Bramness, J.G. Norwegian version of the Mini-International Neuropsychiatric Interview: Feasibility, acceptability and test-retest reliability in an acute psychiatric ward. Eur. Psychiatry 2010, 25, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Piedra, P.D.; Fuentes, G.O.; Gómez, R.H.; Cervantes-villagrana, R.D.; Presno-bernal, J.M.; Elena, L.; Gómez, A. Determinación de Los Intervalos De Referencia de Biometría Hemática en Población Mexicana. Available online: http://www.medigraphic.com/pdfs/patol/pt-2012/pt124j.pdf (accessed on 19 February 2019).
- The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. IPCS, 2009. Available online: http://www.who.int/ipcs/publications/pesticides_hazard_2009.pdf (accessed on 19 February 2019).
- Nagami, H.; Suenaga, T.; Nakazaki, M. Pesticide exposure and subjective symptoms of cut-flower farmers. J. Rural Med. 2017, 12, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achaiyaphum, P.K.; Owteerakul, N.H.; Ujirarat, D.S.; Iri, S.S.; Uwannapong, N.S. Serum Cholinesterase Levels of Thai Chilli-Farm Workers Exposed to Chemical Pesticides: Prevalence Estimates and Associated Factors. J. Occup. Health 2010, 89–98. [Google Scholar] [CrossRef]
- Thetkathuek, A.; Suybros, N.; Daniell, W.; Meepradit, P.; Jaidee, W. Factors Influencing Poisoning Symptoms: A case study of vegetable farmers exposed to mixed insecticides in prek balatchheng village, Cambodia. J. Agromed. 2014, 19, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Singh, P.; Thakur, S.; Dev, B.; Singh, R.; Sunder, S.; Singh, D.; Tazeen, S. Mutation Research/Genetic Toxicology and Environmental Mutagenesis Genetic polymorphisms of GSTM1, GSTT1 and GSTP1 and susceptibility to DNA damage in workers occupationally exposed to organophosphate pesticides. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 725, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Stallones, L.; Beseler, C. Pesticide Illness, Farm Practices, and Neurological Symptoms among Farm Residents in Colorado. Environ. Res. 2002, 97, 89–97. [Google Scholar] [CrossRef]
- Rajkumar, A.P.; Thangadurai, P.; Senthilkumar, P.; Gayathri, K.; Prince, M.; Jacob, K.S. Nature, prevalence and factors associated with depression among the elderly in a rural south Indian community. Int. Psychogeriatr. 2009, 21, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Roh, S. Risk factors associated with depression and suicidal ideation in a rural population. Environ. Health Toxicol. 2016, 31, e2016018. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jung, A.; Yun, D.; Lee, M.; Lee, M.; Choi, Y.; Kim, Y.; Park, C.; Hong, Y.; Kim, S. Association of urinary 3-phenoxybenzoic acid levels with self-reported depression symptoms in a rural elderly population in Asan, South Korea. Environ. Health Toxicol. 2015, e2015002. [Google Scholar] [CrossRef] [PubMed]
- Magauzi, R.; Mabaera, B.; Rusakaniko, S.; Chimusoro, A.; Ndlovu, N.; Tshimanga, M.; Shambira, G.; Chadambuka, A.; Gombe, N. Health effects of agrochemicals among farm workers in commercial farms of Kwekwe district, Zimbabwe. Pan Afr. Med. J. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Lefkowitz, L.J.; Kupina, J.M.; Hirth, N.L.; Henry, R.M.; Noland, G.Y.; Barbee, J.Y.; Zhou, J.Y.; Weese, C.B. Intraindividual stability of human erythrocyte cholinesterase activity. Clin. Chem. 2007, 53, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Freitas Leal, J.K.; Adjobo-Hermans, M.J.W.; Brock, R.; Bosman, G.J.C.G.M. Acetylcholinesterase provides new insights into red blood cell aging in vivo and in vitro. Blood Transfus. 2017, 15, 23–28. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Iglesias, V.P.; Muñoz, M.P.; Cornejo, C.A.; Achu, E.; Baumert, B.; Hanchey, A.; Concha, C.; Brito, A.M.; et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: A review. Int. J. Occup. Environ. Health 2016, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jagodic, H.K.; Agius, M.; Pregelj, P. Inter-Regional Variations in Suicide Rates. Available online: https://pdfs.semanticscholar.org/33e4/5027f82fcb19fbe4c6e242d6a00cbd820010.pdf (accessed on 19 February 2019).
- Handley, T.E.; Inder, K.J.; Kelly, B.J.; Attia, J.R.; Kay-lambkin, F.J. Review Article Urban—Rural influences on suicidality: Gaps in the existing literature and recommendations for future research. Aust. J. Rural Health 2011, 279–283. [Google Scholar] [CrossRef] [PubMed]
- London, L.; Mergler, D.; Kromhout, H. Suicide and Exposure to Organophosphate Insecticides: Cause or Effect? Am. J. Ind. Med. 2005, 321, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Rojas-García, A.E.; Medina-Díaz, I.M.; Robledo-Marenco, M.L.; Barrón-Vivanco, B.S.; Girón-Pérez, M.I.; Velázquez-Fernández, J.B.; González-Arias, C.A.; Albores-Medina, A.; Quintanilla-Vega, B.; Ostrosky-Wegman, P.; et al. Hematological, biochemical effects, and self-reported symptoms in pesticide retailers. J. Occup. Environ. Med. 2011, 53, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Hernández, Y.Y.; Medina-Díaz, I.M.; Barrón-Vivanco, B.S.; Robledo-Marenco, M.L.; Girón-Pérez, M.I.; Pérez-Herrera, N.E.; Quintanilla-Vega, B.; Cerda-Flores, R.; Rojas-García, A.E. Paraoxonase 1 and Its relationship with pesticide biomarkers in indigenous Mexican farmworkers. J. Occup. Environ. Med. 2014, 56, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, J.; Diaz Romo, G.J.; Hesketh, T. Exposure of young children working on Mexican tobacco plantations to organophosphorus and carbamic pesticides, indicated by cholinesterase depression. Child Care Health Dev. 2007, 33, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Santana, M.; Farías-Gómez, C.; Zúñiga-Venegas, L.; Sandoval, R.; Roeleveldo, N.; Van der Velden, K.; Scheepers, P.T.J.; Pancetti, F. Biomonitoring of blood cholinesterases and acyl-peptide hydrolase activities in rural inhabitants exposed to pesticides in the Coquimbo Region of Chile. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, B.N.; Thiermann, H.; Eyer, P.; Worek, F.; Bowe, S.J.; Dawson, A.H.; Buckley, N.A. Evaluation of the test-mate ChE (cholinesterase) field kit in acute organophosphorus poisoning. Ann. Emerg. Med. 2011, 58, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Suratman, S.; Edwards, J.W.; Babina, K. Organophosphate pesticides exposure among farmworkers: Pathways and risk of adverse health effects. Rev. Environ. Health 2015, 30, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Heide, E.A. Case Studies in Environmental Medicine. In Cholinesterase Inhibitors: Including Pesticides and Chemical Warfare Nerve Agents; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007. Available online: https://www.atsdr.cdc.gov/ (accessed on 19 February 2019).
- Rastogi, S.K.; Tripathi, S.; Ravishanker, D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural worker. Indian J. Occup. Environ. Med. 2010, 14, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, S.; Chahal, K.S.; Prakash, A. Potential pharmacological strategies for the improved treatment of organophosphate-induced neurotoxicity. Can. J. Physiol. Pharmacol. 2014, 92, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. Biomed. Res. Int. 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Variable | Nonexposed (n = 100) | Exposed (n = 140) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (Years) | 0.000 | ||||
| 18–34 | 89 | 89.0 | 84 | 60 | |
| 35–50 | 9 | 9.0 | 52 | 37.1 | |
| >51 | 2 | 2.0 | 4 | 2.9 | |
| Gender | 0.751 | ||||
| Female | 60 | 60.0 | 88 | 62.9 | |
| Male | 40 | 40.0 | 52 | 37.1 | |
| Education | 0.000 | ||||
| Illiterate | 0 | 0 | 12 | 8.6 | |
| Elementary school | 1 | 1.0 | 78 | 55.7 | |
| Middle school | 2 | 2.0 | 38 | 27.1 | |
| High school | 0 | 0 | 10 | 7.1 | |
| College | 97 | 97 | 2 | 1.4 | |
| Marital status | 0.000 | ||||
| Single | 60 | 60.0 | 20 | 14.3 | |
| Free union | 35 | 35.0 | 68 | 48.6 | |
| Married | 4 | 4.0 | 48 | 34.3 | |
| Divorced | 1 | 1.0 | 0 | 0 | |
| Widowed | 0 | 0 | 4 | 2.9 | |
| Occupation | 0.000 | ||||
| Farmworker | 20 | 20.0 | 140 | 100.0 | |
| Student | 80 | 80.0 | 0 | 0 | |
| Place of residence in the last six months | 0.000 | ||||
| Provincial center | 100 | 100.0 | 0 | 0 | |
| Surrounding village | 0 | 0 | 140 | 100.0 | |
| Smokers | 23 | 23.0 | 22 | 15.7 | 0.040 |
| Alcohol drinkers | 54 | 54.0 | 24 | 17.4 | 0.000 |
| Abuse substances | 0.190 | ||||
| Cocaine | 3 | 3.0 | 4 | 2.8 | |
| Heroin | 0 | 0 | 3 | 2.1 | |
| Marihuana | 5 | 5.0 | 12 | 8.6 | |
| Crystal | 1 | 1.0 | 11 | 7.9 | |
| No abuse substances | 91 | 91.0 | 110 | 78.6 | |
| Diagnosed psychiatric disorder | 0.000 | ||||
| Depression | 3 | 3.0 | 20 | 14.3 | |
| Major depression with suicidal risk | 8 | 8.0 | 44 | 31.4 | |
| Generalized anxiety | 18 | 18.0 | 20 | 14.3 | |
| Depression–generalized anxiety | 7 | 7.0 | 20 | 14.3 | |
| No psychiatric-disorder diagnosis | 64 | 64.0 | 36 | 25.7 | |
| Parameter | Nonexposed (Mean ± SD) | Exposed (Mean ± SD) | F | p |
|---|---|---|---|---|
| Hb (g/dL) | 21.8 | 0.000 * | ||
| Female | 12.72 ±1.07 | 12.66 ± 2.25 | ||
| Male | 15.15 ± 0.90 | 13.74 ± 2.54 | ||
| AChE (mU/μLHb) | 33.2 | 0.000 * | ||
| Female | 582.43 ± 53.63 | 435.69 ± 103.28 | ||
| Male | 485.39 ± 40.78 | 398.99 ± 84.89 | ||
| AChE activity (mE/min) | 227.95 ± 10.19 | 178.88 ± 37.48 | 126.9 | 0.000 |
| AChE activity (%) | 97.53 ± 4.33 | 76.39 ± 15.93 | 126.9 | 0.000 |
| AChE inhibition (%) | 2.4 ± 4.33 | 23.32 ± 15.92 | 126.9 | 0.000 |
| Pesticide Name | WHO * Classification |
|---|---|
| Azinphos-methyl | IB |
| Chlorpyrifos | III |
| Dicofol | II |
| Malathion | III |
| Methamidophos | IB |
| Methyl parathion | IA |
| Phosmet | II |
| Variable (N) % | Depression Disorder | Suicidal Attempt | Generalized Anxiety | Depression and Generalized Anxiety | No Psychiatric Diagnosis Disorder | p |
|---|---|---|---|---|---|---|
| Weekly pesticide exposure time in hours (N) | ||||||
| 30–60 | (16) 11.4 | (40) 28.6 | (14) 10.0 | (20) 14.3 | (32) 22.9 | 0.045 |
| 61–90 | (4) 2.9 | (4) 2.9 | (6) 4.3 | (4) 2.9 | ||
| Annual pesticide-exposure time in years (N) | ||||||
| 0.5–1 | (12) 8.6 | (22) 15.7 | (12) 8.6 | (10) 7.1 | (22) 15.7 | 0.113 |
| 2–9 | (8) 5.7 | (20) 14.3 | (6) 4.3 | (10) 7.1 | (8) 5.7 | |
| 10–20 | (0)0 | (2) 1.4 | (2) 1.4 | (0) 0 | (6) 4.3 | |
| Neurological symptoms (N) | ||||||
| Shoulder pain | (6) 4.3 | (28) 20 | (2) 1.4 | (14) 10 | (18) 12.9 | 0.001 |
| Back pain | (10) 7.1 | (34) 24.3 | (10) 7.1 | (18) 12.9 | (18) 12.9 | 0.003 |
| Numbness | (6) 4.3 | (24) 17.1 | (8) 5.7 | (14) 10 | (20) 14.3 | 0.095 |
| Nocturia | (4) 2.9 | (18) 12.9 | (6) 4.3 | (6) 4.3 | (14) 10 | 0.504 |
| Dyspnea | (4) 2.9 | (16) 11.4 | (4) 2.9 | (10) 7.1 | (10) 7.1 | 0.175 |
| Insomnia | (2) 1.4 | (12) 8.6 | (4) 2.9 | (8) 5.7 | (10) 7.1 | 0.267 |
| Dizzines | (10) 7.1 | (30) 21.4 | (2) 1.4 | (14) 10 | (16) 11.4 | 0.001 |
| Abdominal discomfort | (8) 5.7 | (23) 16.4 | (0) 0 | (12) 8.6 | (14) 10 | 0.001 |
| Psychiatric Disorders | Acethylcholinesterase Activity Values | |||
|---|---|---|---|---|
| Specific Activity, mU/μLHb (Mean ± SD) | Activity, mE/min (Mean ± SD) | % Activity (Mean ± SD) | % Inhibition (Mean ± SD) | |
| Depression disorder | ||||
| Exposed | 436.49 ± 85.66 | 181.85 ± 22.85 | 77.94 ± 9.70 | 22.06 ± 9.71 |
| Nonexposed | 481.31 ± 117.60 | 208.93 ± 33.71 | 89.45 ± 14.32 | 10.55 ± 14.32 |
| p | 0.377 | 0.057 | 0.057 | 0.057 |
| Major depression with suicidal risk | ||||
| Exposed | 414.34 ± 92.75 | 174.89 ± 32.33 | 74.99 ± 13.74 | 25.01 ± 13.73 |
| Nonexposed | 555.86 ± 55.25 | 228.43 ± 7.27 | 97.73 ± 3.08 | 2.27 ± 3.09 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
| Generalized anxiety | ||||
| Exposed | 388.62 ± 90.04 | 177.48 ± 39.87 | 76.08 ± 16.94 | 23.91 ± 16.94 |
| Nonexposed | 553.44 ± 72.42 | 228.11 ± 7.34 | 97.60 ± 3.12 | 2.340 ± 3.12 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
| Depression and generalized anxiety | ||||
| Exposed | 421.66 ± 106.27 | 178.23 ± 48.70 | 76.40 ± 20.69 | 23.59 ± 20.69 |
| Nonexposed | 566.99 ± 78.36 | 225.71 ± 8.20 | 96.58 ± 3.49 | 3.42 ± 3.49 |
| p | 0.003 | 0.000 | 0.000 | 0.000 |
| Without a psychiatric diagnosis | ||||
| Exposed | 440.25 ± 108.81 | 181.96 ± 42.73 | 76.54 ± 15.93 | 22.01 ± 18.15 |
| Nonexposed | 539.75 ± 63.44 | 229.28 ± 7.65 | 98.09 ± 3.25 | 1.91 ± 3.25 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Medina, A.; Ugalde-Lizárraga, A.; Bojorquez-Cuevas, M.S.; Garnica-Ruiz, J.; González-Corral, M.A.; García-Ledezma, A.; Pineda-García, G.; Cornejo-Bravo, J.M. Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México. Int. J. Environ. Res. Public Health 2019, 16, 689. https://doi.org/10.3390/ijerph16050689
Serrano-Medina A, Ugalde-Lizárraga A, Bojorquez-Cuevas MS, Garnica-Ruiz J, González-Corral MA, García-Ledezma A, Pineda-García G, Cornejo-Bravo JM. Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México. International Journal of Environmental Research and Public Health. 2019; 16(5):689. https://doi.org/10.3390/ijerph16050689
Chicago/Turabian StyleSerrano-Medina, Aracely, Angel Ugalde-Lizárraga, Michelle Stephanie Bojorquez-Cuevas, Jatniel Garnica-Ruiz, Martín Alexis González-Corral, Arnold García-Ledezma, Gisela Pineda-García, and José Manuel Cornejo-Bravo. 2019. "Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México" International Journal of Environmental Research and Public Health 16, no. 5: 689. https://doi.org/10.3390/ijerph16050689
APA StyleSerrano-Medina, A., Ugalde-Lizárraga, A., Bojorquez-Cuevas, M. S., Garnica-Ruiz, J., González-Corral, M. A., García-Ledezma, A., Pineda-García, G., & Cornejo-Bravo, J. M. (2019). Neuropsychiatric Disorders in Farmers Associated with Organophosphorus Pesticide Exposure in a Rural Village of Northwest México. International Journal of Environmental Research and Public Health, 16(5), 689. https://doi.org/10.3390/ijerph16050689