Bronchodilator Response Predicts Longitudinal Improvement in Small Airway Function in World Trade Center Dust Exposed Community Members
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Procedures
2.3. Spirometry and FOT Measurements
2.4. Definitions
2.5. Statistical Methods
3. Results
3.1. Patient Demographics and Characteristics
3.2. Lung Function at Initial Visit
3.3. Longitudinal Spirometry Measurements
3.4. Longitudinal FOT Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reibman, J.; Lin, S.; Hwang, S.A.; Gulati, M.; Bowers, J.A.; Rogers, L.; Berger, K.I.; Hoerning, A.; Gomez, M.; Fitzgerald, E.F. The World Trade Center residents’ respiratory health study: New-onset respiratory symptoms and pulmonary function. Environ. Health Perspect. 2005, 113, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Reibman, J.; Liu, M.; Cheng, Q.; Liautaud, S.; Rogers, L.; Lau, S.; Berger, K.I.; Goldring, R.M.; Marmor, M.; Fernandez-Beros, M.E.; et al. Characteristics of a Residential and Working Community With Diverse Exposure to World Trade Center Dust, Gas, and Fumes. J. Occup. Environ. Med. 2009, 51, 234–541. [Google Scholar] [CrossRef] [PubMed]
- Caplan-Shaw, C.; Kazeros, A.; Pradhan, D.; Berger, K.; Goldring, R.; Zhao, S.; Liu, M.; Shao, Y.; Fernandez-Beros, M.E.; Marmor, M.; et al. Improvement in severe lower respiratory symptoms and small airway function in World Trade Center dust exposed community members. Am. J. Ind. Med. 2016, 59, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.M.; Maslow, C.B.; Reibman, J.; Pillai, P.S.; Goldring, R.M.; Farfel, M.R.; Stellman, S.D.; Berger, K.I. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am. J. Respir. Crit. Care Med. 2011, 184, 582–589. [Google Scholar] [CrossRef]
- Jordan, H.T.; Friedman, S.M.; Reibman, J.; Goldring, R.M.; Miller Archie, S.A.; Ortega, F.; Alper, H.; Shao, Y.; Maslow, C.B.; Cone, J.E.; et al. Risk factors for persistence of lower respiratory symptoms among community members exposed to the 2001 World Trade Center terrorist attacks. Occup. Environ. Med. 2017, 74, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prezant, D.J.; Weiden, M.; Banauch, G.I.; McGuinness, G.; Rom, W.N.; Aldrich, T.K.; Kelly, K.J. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N. Engl. J. Med. 2002, 347, 806–815. [Google Scholar] [CrossRef]
- Webber, M.P.; Gustave, J.; Lee, R.; Niles, J.K.; Kelly, K.; Cohen, H.W.; Prezant, D.J. Trends in respiratory symptoms of firefighters exposed to the world trade center disaster: 2001–2005. Environ. Health Perspect. 2009, 117, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.; Moline, J.; Skloot, G.; Metzger, K.; Baron, S.; Luft, B.; Markowitz, S.; Udasin, I.; Harrison, D.; Stein, D.; et al. The world Trade Center Disaster and the Health of Workers: Five-Year Assessment of a Unique Medical Screening Program. Environ. Health Perspect. 2006, 114, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Maslow, C.B.; Friedman, S.M.; Pillai, P.S.; Reibman, J.; Berger, K.I.; Goldring, R.; Stellman, S.D.; Farfel, M. Chronic and acute exposures to the world trade center disaster and lower respiratory symptoms: Area residents and workers. Am. J. Public Health 2012, 102, 1186–1194. [Google Scholar] [CrossRef]
- Niles, J.K.; Webber, M.P.; Cohen, H.W.; Hall, C.B.; Zeig-Owens, R.; Ye, F.; Glaser, M.S.; Weakley, J.; Weiden, M.D.; Aldrich, T.K.; et al. The respiratory pyramid: From symptoms to disease in World Trade Center exposed firefighters. Am. J. Ind. Med. 2013, 56, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, B.W.; Goldring, R.M.; Herberg, M.E.; Hofer, I.S.; Reyfman, P.A.; Liautaud, S.; Rom, W.N.; Reibman, J.; Berger, K.I. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007, 132, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Weiden, M.D.; Kwon, S.; Caraher, E.; Berger, K.I.; Reibman, J.; Rom, W.N.; Prezant, D.J.; Nolan, A. Biomarkers of World Trade Center Particulate Matter Exposure: Physiology of Distal Airway and Blood Biomarkers that Predict FEV(1) Decline. Semin. Respir. Crit. Care Me. 2015, 36, 323–333. [Google Scholar] [CrossRef] [PubMed]
- de la Hoz, R.E.; Liu, X.; Doucette, J.T.; Reeves, A.P.; Bienenfeld, L.A.; Wisnivesky, J.P.; Celedon, J.C.; Lynch, D.A.; San Jose Estepar, R. Increased Airway Wall Thickness is Associated with Adverse Longitudinal First-Second Forced Expiratory Volume Trajectories of Former World Trade Center workers. Lung 2018, 196, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qian, M.; Cheng, Q.; Berger, K.I.; Shao, Y.; Turetz, M.; Kazeros, A.; Parsia, S.; Goldring, R.M.; Caplan-Shaw, C.; et al. Longitudinal spirometry among patients in a treatment program for community members with World Trade Center-related illness. J. Occup. Environ. Med. 2012, 54, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Zeig-Owens, R.; Singh, A.; Aldrich, T.K.; Hall, C.B.; Schwartz, T.; Webber, M.P.; Cohen, H.W.; Kelly, K.J.; Nolan, A.; Prezant, D.J.; et al. Blood Leukocyte Concentrations, FEV1 Decline, and Airflow Limitation. A 15-Year Longitudinal Study of World Trade Center-exposed Firefighters. Ann. Am. Thorac. Soc. 2018, 15, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Caplan-Shaw, C.E.; Yee, H.; Rogers, L.; Abraham, J.L.; Parsia, S.S.; Naidich, D.P.; Borczuk, A.; Moreira, A.; Shiau, M.C.; Ko, J.P.; et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J. Occup. Environ. Med. 2011, 53, 981–991. [Google Scholar] [CrossRef]
- Kazeros, A.; Zhang, E.; Cheng, X.; Shao, Y.; Liu, M.; Qian, M.; Caplan-Shaw, C.; Berger, K.I.; Goldring, R.M.; Ghumman, M.; et al. Systemic Inflammation Associated With World Trade Center Dust Exposures and Airway Abnormalities in the Local Community. J. Occup. Environ. Med. 2015, 57, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, K.I.; Turetz, M.; Liu, M.; Shao, Y.; Kazeros, A.; Parsia, S.; Caplan-Shaw, C.; Friedman, S.M.; Maslow, C.B.; Marmor, M.; et al. Oscillometry complements spirometry in evaluation of subjects following toxic inhalation. ERJ Open Res. 2015, 1. [Google Scholar] [CrossRef]
- Mead, J. The lung’s “quiet zone”. N. Engl. J. Med. 1970, 282, 1318–1319. [Google Scholar] [CrossRef]
- Macklem, P.T. The physiology of small airways. Am. J. Respir. Crit. Care Med. 1998, 157, S181–S183. [Google Scholar] [CrossRef]
- Pedley, T.J.; Schroter, R.C.; Sudlow, M.F. The prediction of pressure drop and variation of resistance within the human bronchial airways. Respir. Physiol. 1970, 9, 387–405. [Google Scholar] [CrossRef]
- Fredberg, J.J.; Mead, J. Impedance of intrathoracic airway models during low-frequency periodic flow. J. Appl. Physiol. 1979, 47, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.H.; Lutchen, K.R. The interface between measurement and modeling of peripheral lung mechanics. Respir. Physiol. Neurobiol. 2005, 148, 153–164. [Google Scholar] [CrossRef]
- Goldman, M.D.; Saadeh, C.; Ross, D. Clinical applications of forced oscillation to assess peripheral airway function. Respir. Physiol. Neurobiol. 2005, 148, 179–194. [Google Scholar] [CrossRef]
- Bateman, E.D.; Hurley, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, J.M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2018, 51, 143–178. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farre, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, B.W.; Goldring, R.M.; Berger, K.I. Distal airway function assessed by oscillometry at varying respiratory rate: Comparison with dynamic compliance. COPD 2009, 6, 162–170. [Google Scholar] [CrossRef]
- Kjeldgaard, J.M.; Hyde, R.W.; Speers, D.M.; Reichert, W.W. Frequency dependence of total respiratory resistance in early airway disease. Am. Rev. Respir. Dis. 1976, 114, 501–508. [Google Scholar] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oostveen, E.; Boda, K.; van der Grinten, C.P.; James, A.L.; Young, S.; Nieland, H.; Hantos, Z. Respiratory impedance in healthy subjects: Baseline values and bronchodilator response. Eur. Respir. J. 2013, 42, 1513–1523. [Google Scholar] [CrossRef]
- Mendelson, D.S.; Roggeveen, M.; Levin, S.M.; Herbert, R.; de la Hoz, R.E. Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic World Trade Center rescue and recovery workers. J. Occup. Environ. Med. 2007, 49, 840–845. [Google Scholar] [CrossRef]
- Weiden, M.D.; Ferrier, N.; Nolan, A.; Rom, W.N.; Comfort, A.; Gustave, J.; Zeig-Owens, R.; Zheng, S.; Goldring, R.M.; Berger, K.I.; et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest 2010, 137, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Brauer, M.; del Carmen, A.; Fortoul, T.I.; Wright, J.L.; Churg, A.; Brauer, M.; Fortoul, T.I.; Wright, J.L. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect. 2003, 111, 714–718. [Google Scholar] [CrossRef]
- Guerry-Force, M.L.; Muller, N.L.; Wright, J.L.; Wiggs, B.; Coppin, C.; Pare, P.D.; Hogg, J.C. A comparison of bronchiolitis obliterans with organizing pneumonia, usual interstitial pneumonia, and small airways disease. Am. Rev. Respir. Dis. 1987, 135, 705–712. [Google Scholar]
- Chia, K.S.; Ng, T.P.; Jeyaratnam, J. Small airways function of silica-exposed workers. Am. J. Ind. Med. 1992, 22, 155–162. [Google Scholar] [CrossRef]
- Mann, J.M.; Sha, K.K.; Kline, G.; Breuer, F.U.; Miller, A. World Trade Center dyspnea: Bronchiolitis obliterans with functional improvement: A case report. Am. J. Ind. Med. 2005, 48, 225–229. [Google Scholar] [CrossRef]
- Rice, M.B.; Rifas-Shiman, S.L.; Litonjua, A.A.; Oken, E.; Gillman, M.W.; Kloog, I.; Luttmann-Gibson, H.; Zanobetti, A.; Coull, B.A.; Schwartz, J.; et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am. J. Respir. Crit. Care Med. 2016, 193, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Dorans, K.S.; Gold, D.R.; Schwartz, J.; Koutrakis, P.; Washko, G.R.; O’Connor, G.T.; Mittleman, M.A. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am. J. Respir. Crit. Care Med. 2015, 191, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Skloot, G.; Goldman, M.; Fischler, D.; Goldman, C.; Schechter, C.; Levin, S.; Teirstein, A. Respiratory symptoms and physiologic assessment of ironworkers at the World Trade Center disaster site. Chest 2004, 125, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.I.; Reibman, J.; Oppenheimer, B.W.; Vlahos, I.; Harrison, D.; Goldring, R.M. Lessons from the world trade center disaster: Airway disease presenting as restrictive dysfunction. Chest 2013, 144, 249–257. [Google Scholar] [CrossRef]
- van den Elshout, F.J.; van Herwaarden, C.L.; Folgering, H.T. Oscillatory respiratory impedance and lung tissue compliance. Respir. Med. 1994, 88, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Otis, A.B.; McKerrow, C.B.; Bartlett, R.A.; Mead, J.; McIlroy, M.B.; Selverstone, N.J.; Radford, E.P. Mechanical factors in distribution of pulmonary ventilation. J. Appl. Physiol. 1956, 8, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Woolcock, A.J.; Read, J. Lung volumes in exacerbations of asthma. Am. J. Med. 1966, 41, 259–273. [Google Scholar] [CrossRef]
- Ayres, S.M.; Griesbach, S.J.; Reimold, F.; Evans, R.G. Bronchial component in chronic obstructive lung disease. Am. J. Med. 1974, 57, 183–191. [Google Scholar] [CrossRef]
- Ramsdell, J.W.; Tisi, G.M. Determination of bronchodilation in the clinical pulmonary function laboratory. Role of changes in static lung volumes. Chest 1979, 76, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Rodarte, J.R.; Brusasco, V. Assessing the reversibility of airway obstruction. Chest 1998, 114, 1607–1612. [Google Scholar] [CrossRef]
- Newton, M.F.; O’Donnell, D.E.; Forkert, L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest 2002, 121, 1042–1050. [Google Scholar] [CrossRef]
- Brackbill, R.M.; Hadler, J.L.; DiGrande, L.; Ekenga, C.C.; Farfel, M.R.; Friedman, S.; Perlman, S.E.; Stellman, S.D.; Walker, D.J.; Wu, D.; et al. Asthma and posttraumatic stress symptoms 5 to 6 years following exposure to the World Trade Center terrorist attack. JAMA 2009, 302, 502–516. [Google Scholar] [CrossRef]
- Jordan, H.T.; Stellman, S.D.; Reibman, J.; Farfel, M.R.; Brackbill, R.M.; Friedman, S.M.; Li, J.; Cone, J.E. Factors associated with poor control of 9/11-related asthma 10-11 years after the 2001 World Trade Center terrorist attacks. J. Asthma 2015, 52, 630–637. [Google Scholar] [CrossRef]
- Webber, M.P.; Glaser, M.S.; Weakley, J.; Soo, J.; Ye, F.; Zeig-Owens, R.; Weiden, M.D.; Nolan, A.; Aldrich, T.K.; Kelly, K.; et al. Physician-diagnosed respiratory conditions and mental health symptoms 7-9 years following the World Trade Center disaster. Am. J. Ind. Med. 2011, 54, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Wisnivesky, J.P.; Teitelbaum, S.L.; Todd, A.C.; Boffetta, P.; Crane, M.; Crowley, L.; de la Hoz, R.E.; Dellenbaugh, C.; Harrison, D.; Herbert, R.; et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: A cohort study. Lancet 2011, 378, 888–897. [Google Scholar] [CrossRef]
- Kim, H.; Herbert, R.; Landrigan, P.; Markowitz, S.B.; Moline, J.M.; Savitz, D.A.; Todd, A.C.; Udasin, I.G.; Wisnivesky, J.P. Increased rates of asthma among World Trade Center disaster responders. Am. J. Ind. Med. 2012, 55, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kazeros, A.; Maa, M.T.; Patrawalla, P.; Liu, M.; Shao, Y.; Qian, M.; Turetz, M.; Parsia, S.; Caplan-Shaw, C.; Berger, K.I.; et al. Elevated peripheral eosinophils are associated with new-onset and persistent wheeze and airflow obstruction in world trade center-exposed individuals. J. Asthma 2013, 50, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Rom, W.N.; Weiden, M.; Garcia, R.; Yie, T.A.; Vathesatogkit, P.; Tse, D.B.; McGuinness, G.; Roggli, V.; Prezant, D. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am. J. Respir. Crit. Care Med. 2002, 166, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Fireman, E.M.; Lerman, Y.; Ganor, E.; Greif, J.; Fireman-Shoresh, S.; Lioy, P.J.; Banauch, G.I.; Weiden, M.; Kelly, K.J.; Prezant, D.J. Induced sputum assessment in New York City firefighters exposed to World Trade Center dust. Environ. Health Perspect. 2004, 112, 1564–1569. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Age, median (IQR) | 51 (44–59) |
| Gender; (M/F, %) | 50/50 |
| BMI (kg/m2), median (IQR) | 28 (25–32) |
| Race/Ethnicity | |
| Hispanic | 268 (36) |
| White | 241 (33) |
| Black | 155 (21) |
| Asian | 52 (7) |
| Unspecified | 25 (3) |
| Income/year ≤ $15,000 | 286 (39) |
| Smoking history | |
| >5 pack-year | 177 (24) |
| Current smoker | 96 (13) |
| WTC exposure category | |
| Local worker | 378 (51) |
| Clean up worker | 128 (17) |
| Resident | 149 (20) |
| Rescue/recovery | 25 (3) |
| Unspecified | 61 (8) |
| Caught in WTC dust cloud | 382 (52) |
| Lower respiratory symptoms | 691 (93) |
| Pre-BD | Post-BD | |
|---|---|---|
| Spirometry | ||
| FVC (%predicted) | 92 (81–103) | 92 (81–103) |
| FEV1 (%predicted) | 88 (76–99) | 92 (80–103) |
| FEV1/FVC | 77 (71–81) | 79 (74–84) |
| FOT | ||
| R5 (kPa/L/s) | 0.489 (0.375–0.615) | 0.428 (0.328–0.527) |
| R5–20 (kPa/L/s) | 0.098 (0.055–0.168) | 0.074 (0.041–0.128) |
| Whole Cohort | Abnormal Spirometry | |||
|---|---|---|---|---|
| (n = 741) | Total (n = 270) | (−) BD Response (n = 212) | (+) BD Response (n = 58) | |
| ΔFVC (mL/year) | 36 ± 6 * | 43 ± 10 * | 28 ± 9 ** | 122 ± 28 * |
| ΔFEV1 (mL /year) | 22 ± 4 * | 29 ± 7 * | 16 ± 7 ** | 91 ± 7 * |
| Whole Cohort | Abnormal Oscillometry | |||
|---|---|---|---|---|
| (n = 741) | Total (n = 527) | (−) BD Response (n = 364) | (+) BD Response (n = 163) | |
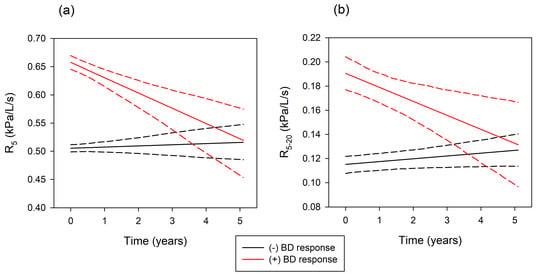
| ΔR5 (kPa/L/s/year) | −0.001 ± 0.002 | −0.006 ± 0.003 * | 0.002 ± 0.003 * | −0.027 ± 0.006 ** |
| ΔR5–20 (kPa/L/s/year) | 0.001 ± 0.001 | −0.001 ± 0.001 | 0.002 ± 0.001 | −0.012 ± 0.004 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, D.; Xu, N.; Reibman, J.; Goldring, R.M.; Shao, Y.; Liu, M.; Berger, K.I. Bronchodilator Response Predicts Longitudinal Improvement in Small Airway Function in World Trade Center Dust Exposed Community Members. Int. J. Environ. Res. Public Health 2019, 16, 1421. https://doi.org/10.3390/ijerph16081421
Pradhan D, Xu N, Reibman J, Goldring RM, Shao Y, Liu M, Berger KI. Bronchodilator Response Predicts Longitudinal Improvement in Small Airway Function in World Trade Center Dust Exposed Community Members. International Journal of Environmental Research and Public Health. 2019; 16(8):1421. https://doi.org/10.3390/ijerph16081421
Chicago/Turabian StylePradhan, Deepak, Ning Xu, Joan Reibman, Roberta M. Goldring, Yongzhao Shao, Mengling Liu, and Kenneth I. Berger. 2019. "Bronchodilator Response Predicts Longitudinal Improvement in Small Airway Function in World Trade Center Dust Exposed Community Members" International Journal of Environmental Research and Public Health 16, no. 8: 1421. https://doi.org/10.3390/ijerph16081421
APA StylePradhan, D., Xu, N., Reibman, J., Goldring, R. M., Shao, Y., Liu, M., & Berger, K. I. (2019). Bronchodilator Response Predicts Longitudinal Improvement in Small Airway Function in World Trade Center Dust Exposed Community Members. International Journal of Environmental Research and Public Health, 16(8), 1421. https://doi.org/10.3390/ijerph16081421