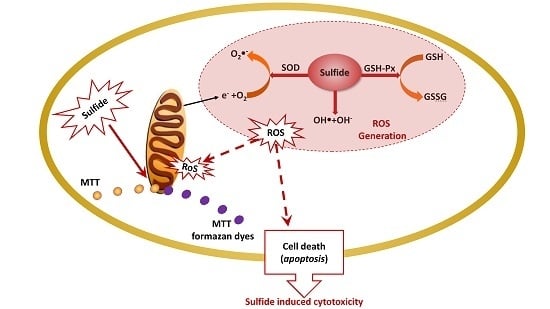
Oxidative Stress Effects of Soluble Sulfide on Human Hepatocyte Cell Line LO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. The MTT Assay
2.4. Measurement of the Hydroxyl Radical
2.5. Measurement of SOD Activity
2.6. Measurement of GSH-Px Activity
2.7. Statistical Analysis
3. Results
3.1. Cytotoxic Effects of Soluble Sulfide
3.2. Oxidative Stress Effects of Soluble Sulfide
3.2.1. Hydroxyl Radical Production by Soluble Sulfide
3.2.2. SOD and GSH-Px Activities Induction by Soluble Sulfide
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petruci, J.F.D.S.; Cardoso, A.A. A new palladium chelate compound for determination of sulfide. Microchem. J. 2013, 106, 368–372. [Google Scholar] [CrossRef]
- Lin, H.W.; Lu, Y.; Ganigué, R.; Sharma, K.R.; Rabaey, K.; Yuan, Z.; Pikaar, I. Simultaneous use of caustic and oxygen for efficient sulfide control in sewers. Sci. Total Environ. 2017, 601–602, 776–783. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, L.L.; Gao, H.W.; Wang, F. Effect of Soluble Sulfide on the Activity of Luminescent Bacteria. Molecules 2012, 17, 6046–6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonobe, T.; Haouzi, P. Sulfide Intoxication-Induced Circulatory Failure is Mediated by a Depression in Cardiac Contractility. Cardiovasc. Toxicol. 2016, 16, 67–78. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Sutherland-Stacey, L.; Corrie, S.; Neethling, A.; Johnson, I.; Gutierrez, O.; Dexter, R.; Yuan, Z.; Keller, J.; Hamilton, G. Continuous measurement of dissolved sulfide in sewer systems. Water Sci. Technol. 2008, 57, 375. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef]
- Wang, R. Gasotransmitters: Growing pains and joys. Trends Biochem. Sci. 2014, 39, 227–232. [Google Scholar] [CrossRef]
- Lefer, D.J. A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2007, 104, 17907–17908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Science Foundation. European Network on Gasotransmitters; European Science Foundation: Strasbourg, France, 2011. [Google Scholar]
- Di Villa Bianca, R.D.E.; Sorrentino, R.; Maffia, P.; Mirone, V.; Imbimbo, C.; Fusco, F.; De Palma, R.; Ignarro, L.J.; Cirino, G. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc. Natl. Acad. Sci. USA 2009, 106, 4513–4518. [Google Scholar] [CrossRef] [Green Version]
- Dominy, J.E.; Stipanuk, M.H. New roles for cysteine and transsulfuration enzymes: Production of H2S, a neuromodulator and smooth muscle relaxant. Nutr. Rev. 2010, 62, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.U.D.; Sattar, M.A.; Rathore, H.A.; Abdullah, M.H.; Tan, S.; Abdullah, N.A.; Johns, E.J. Exogenous Hydrogen Sulfide (H2S) Reduces Blood Pressure and Prevents the Progression of Diabetic Nephropathy in Spontaneously Hypertensive Rats. Ren. Fail. 2012, 34, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P. H2S, a new neuromodulator. Med. Sci. 2004, 20, 697–700. [Google Scholar]
- Yan, S.-K.; Chang, T.; Wang, H.; Wu, L.; Wang, R.; Meng, Q.H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 351, 485–491. [Google Scholar] [CrossRef]
- Lan, A.; Liao, X.; Mo, L.; Yang, C.; Yang, Z.; Wang, X.; Hu, F.; Chen, P.; Feng, J.; Zheng, D. Hydrogen sulfide protects against chemical hypoxia-induced injury by inhibiting ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells. PLoS ONE 2011, 6, e25921. [Google Scholar] [CrossRef]
- Wang, Y.L.; Jia, J.; Ao, G.Z.; Hu, L.F.; Liu, H.; Xiao, Y.Q.; Du, H.P.; Alkayed, N.J.; Liu, C.F.; Cheng, J. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J. Neurochem. 2014, 129, 827–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Cheung, N.S.; Zhu, Y.-Z.; Chu, S.H.; Siau, J.L.; Wong, B.S.; Armstrong, J.S.; Moore, P.K. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 2005, 326, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Zhang, L.; Guo, J.M.; Cao, Y.D.; Shang, E.S.; Tang, Y.P.; Ding, A.W.; Duan, J.A. Processing of kansui roots stir-baked with vinegar reduces kansui-induced hepatocyte cytotoxicity by decreasing the contents of toxic terpenoids and regulating the cell apoptosis pathway. Molecules 2014, 19, 7237–7254. [Google Scholar] [CrossRef]
- Wu, L.; Ying, S.; Hu, Z.; Gao, H. Effects of soluble sulfide on zebrafish (Danio rerio) embryonic development. Environ. Toxicol. Pharm. 2016, 42, 183–189. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating toxicity potential of drug-nanoparticles. Nanopart. Drug Deliv. 2012, 64, 123–155. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays—Assay Guidance Manual; Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004.
- Hayyan, M.; Hashim, M.A.; Alnashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2017, 116, 3029–3085. [Google Scholar] [CrossRef]
- Luo, J.; Nikolaev, A.Y.; Imai, S.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative Control of p53 by Sir2α Promotes Cell Survival under Stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Lu, Z.; Tao, L.; Yang, L.; Guo, Y.; Yang, Y.; Sun, X.; Ding, Q. ROS-Dependent Neuroprotective Effects of NaHS in Ischemia Brain Injury Involves the PARP/AIF Pathway. Cell. Physiol. Biochem. 2015, 36, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, Y.; Hu, Y.; Luo, Y.; Lu, X.; Tsui, C.K.; Lu, L.; Liang, X. Madecassic Acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed. Pharmacother. 2016, 84, 845–852. [Google Scholar] [CrossRef]
- Tang, J.; Lei, C.; Qiang, L.; Wang, L.; Gang, J.; Liu, G.; Chen, X.; Cai, J.; Shang, H.; Hua, Z. Selenoprotein X Gene Knockdown Aggravated H2O2-Induced Apoptosis in Liver LO2 Cells. Biol. Trace Elem. Res. 2016, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Storch, A.; Burkhardt, K.; Ludolph, A.C.; Schwarz, J. Protective effects of riluzole on dopamine neurons: Involvement of oxidative stress and cellular energy metabolism. J. Neurochem. 2000, 75, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Balog, M.; Mark, L.; Montsko, G.; Turi, Z.; Jr, F.G.; Sumegi, B.; Kalai, T.; Hideg, K.; Kovacs, K. Induction of mitochondrial destabilization and necrotic cell death by apolar mitochondria-directed SOD mimetics. Mitochondrion 2011, 11, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Woolliams, J.; Wiener, G.; Anderson, P.; McMurray, C. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res. Vet. Sci. 1983, 34, 253. [Google Scholar] [CrossRef]
- Lin, M.T.; M Flint, B. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158. [Google Scholar]
- Ortega, J.; Ortega, J.; Julian, D. Hypotaurine and sulfhydryl-containing antioxidants reduce H2S toxicity in erythrocytes from a marine invertebrate. J. Exp. Biol. 2008, 211, 3816–3825. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, R.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenergy Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Eghbal, M.A.; Pennefather, P.S.; O’Brien, P.J. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 2004, 203, 69–76. [Google Scholar] [CrossRef]
- Truong, D.H.; Eghbal, M.A.; Hindmarsh, W.; Roth, S.H.; O’Brien, P.J. Molecular Mechanisms of Hydrogen Sulfide Toxicity. Drug Metab. Rev. 2006, 38, 733–744. [Google Scholar] [CrossRef]
- Kombian, S.B.; Reiffenstein, R.; Colmers, W.F. The actions of hydrogen sulfide on dorsal raphe serotonergic neurons in vitro. J. Neurophysiol. 1993, 70, 81–96. [Google Scholar] [CrossRef]
- Ahlman, K.; Koskela, R.S.; Kuikka, P.; Koponen, M.; Annanmaki, M. Mortality among sulfide ore miners. Am. J. Ind. Med. 1991, 19, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2010, 3, 3–8. [Google Scholar]
- Buonocore, G.; Perrone, S.; Tataranno, M.L. Oxygen toxicity: Chemistry and biology of reactive oxygen species. Semin. Fetal Neonatal Med. 2010, 15, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Zhang, J.; Yu, H.; Wang, X.; Wu, J.; Xue, Y. Hydroxyl radical generation and oxidative stress in Carassius auratus liver, exposed to pyrene. Ecotoxicol. Environ. Saf. 2008, 71, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Yan, Z.; Zhao, B.L. Superoxide anion, uncoupling proteins and Alzheimer’s disease. J. Clin. Biochem. Nutr. 2010, 46, 187–194. [Google Scholar] [CrossRef]
- Sun, W.H.; Liu, F.; Chen, Y.; Zhu, Y.C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012, 421, 164–169. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; Deleon, E.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of Hydrogen Sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by Superoxide Dismutase. Redox Biol. 2018, 15, 74–85. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- La Russa, D.; Brunelli, E.; Pellegrino, D. Oxidative imbalance and kidney damage in spontaneously hypertensive rats: Activation of extrinsic apoptotic pathways. Clin. Sci. 2017, 131, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Simic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Mimic-Oka, J. Glutathione S-transferases in kidney and urinary bladder tumors. Nat. Rev. Urol. 2009, 6, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Whitson, J.A.; Wilmarth, P.A.; Klimek, J.; Monnier, V.M.; David, L.; Fan, X. Proteomic analysis of the glutathione-deficient LEGSKO mouse lens reveals activation of EMT signaling, loss of lens specific markers, and changes in stress response proteins. Free Radic. Biol. Med. 2017, 113, 84–96. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Liu, X.; Pan, L.; Xiong, Q.; Zhu, Y.Z. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide 2015, 46, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.; Fridovich, I. Inactivation of glutathione peroxidase by superoxide radical. Arch. Biochem. Biophys. 1985, 240, 500–508. [Google Scholar] [CrossRef]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.-S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Shimada, S.; Fukai, M.; Wakayama, K.; Ishikawa, T.; Kobayashi, N.; Kimura, T.; Yamashita, K.; Kamiyama, T.; Shimamura, T.; Taketomi, A.; et al. Hydrogen sulfide augments survival signals in warm ischemia and reperfusion of the mouse liver. Surg. Today 2015, 45, 892–903. [Google Scholar] [CrossRef]
- Iwayama, K.; Kimura, J.; Mishima, A.; Kusakabe, A.; Ohtaki, K.; Tampo, Y.; Hayase, N. Low concentrations of clarithromycin upregulate cellular antioxidant enzymes and phosphorylation of extracellular signal-regulated kinase in human small airway epithelial cells. J. Pharm. Health Care Sci. 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, Z.P.; Wang, Z.Q.; Chang, J.; Zhang, T.; Chi, Y.Q.; Han, N.; Zhao, K.X. Pyridoxamine Treatment of HK-2 Human Proximal Tubular Epithelial Cells Reduces Oxidative Stress and the Inhibition of Autophagy induced by High Glucose Levels. Med. Sci. Monit. 2019, 25, 1480–1488. [Google Scholar] [CrossRef]
- Jiao, Z.H.; Li, M.; Feng, Y.X.; Shi, J.C.; Zhang, J.; Shao, B. Hormesis Effects of Silver Nanoparticles at Non-Cytotoxic Doses to Human Hepatoma Cells. PLoS ONE 2014, 9, e102564. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Chen, Z.; Wu, L. Oxidative Stress Effects of Soluble Sulfide on Human Hepatocyte Cell Line LO2. Int. J. Environ. Res. Public Health 2019, 16, 1662. https://doi.org/10.3390/ijerph16091662
Shao Y, Chen Z, Wu L. Oxidative Stress Effects of Soluble Sulfide on Human Hepatocyte Cell Line LO2. International Journal of Environmental Research and Public Health. 2019; 16(9):1662. https://doi.org/10.3390/ijerph16091662
Chicago/Turabian StyleShao, Ying, Zhongli Chen, and Lingling Wu. 2019. "Oxidative Stress Effects of Soluble Sulfide on Human Hepatocyte Cell Line LO2" International Journal of Environmental Research and Public Health 16, no. 9: 1662. https://doi.org/10.3390/ijerph16091662
APA StyleShao, Y., Chen, Z., & Wu, L. (2019). Oxidative Stress Effects of Soluble Sulfide on Human Hepatocyte Cell Line LO2. International Journal of Environmental Research and Public Health, 16(9), 1662. https://doi.org/10.3390/ijerph16091662