Premature Aging in Chronic Kidney Disease: The Outcome of Persistent Inflammation beyond the Bounds
Abstract
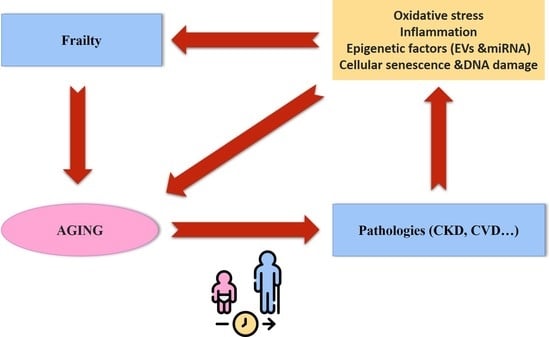
:1. Introduction
Aging: A Physiological Stage Associated with a Higher Frequency of Pathologies
2. Frailty and Pathologies Associated with Aging
| Test | Description | Reference |
|---|---|---|
| Frailty phenotype (Fried criteria)/Cardiovascular health study | Unintentional weight loss >4.5 kg in the last year Weakness (low grip strength) Fatigue and low resistance Slowness Low physical capacity | [41] |
| Frailty index of accumulative deficits | ≥30 symptoms, disease, disabilities, comorbidities, or health deficiencies Expressed as a ratio (for example 3/30 = 0.1) | [37] |
| Vulnerable elders survey | 13 questions about age, self-perceptions of health, needing assistance in daily activities, and physical ability A patient with a score of ≥3 is considered vulnerable | [42] |
| Sarcopenia (loss of muscle due to aging) | Rectus femoris cross-sectional area by ultrasound Computed tomography of the left and right psoas muscles at the L4 vertebra | [43,44] |
| Frailty index derived from the comprehensive geriatric assessment | Clinical analysis of medical, nutritional, functional, and psychological variables Initially 10 domains, but later expanded to 52 domains | [45,46] |
| Edmonton frailty scale | Evaluation of 17 variables on cognition, general health status, self-reported health, functional independence, social support, polypharmacy, mood, continence, and functional performance A person with a score >5 is considered frail, with different severity depending on the score: vulnerable (6–7), mildly frail (8–9), moderately frail (10–11), and severely frail (12–17) | [47] |
| Tilburg frailty indicator | 15 self-reported items evaluating: physical components (weight loss, balance, difficulty in walking, health, gripping, vision, and tiredness), psychological factors (memory, anxiety, coping mechanisms and feeling down), social elements (living conditions, social isolation, social support) A person with a score of ≥5 is considered frail | [48] |
3. Chronic Kidney Disease
3.1. Brief Description of Chronic Kidney Disease
3.2. Chronic Kidney Disease during the Process of Physiological Aging
4. Premature Aging in Chronic Kidney Disease: A Process Associated with an Increase in Cardiovascular Pathologies
5. Traditional Factors Involved in Accelerated Aging Induced by Chronic Kidney Disease
5.1. Oxidative Stress
5.2. Inflammation
6. Novel Factors Implied in Accelerated Aging Induced by Chronic Kidney Disease
6.1. Epigenetic Factors: Extracellular Vesicles and microRNAs
6.2. Genomic Damage and Cellular Senescence Induced by Chronic Kidney Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitnitski, A.; Rockwood, K. Aging as a Process of Deficit Accumulation: Its Utility and Origin. Interdiscip. Top. Gerontol. 2015, 40, 85–98. [Google Scholar] [CrossRef]
- Oeppen, J. DEMOGRAPHY: Enhanced: Broken Limits to Life Expectancy. Science 2002, 296, 1029–1031. [Google Scholar] [CrossRef] [Green Version]
- Luy, M.; Di Giulio, P.; di Lego, V.; Lazarevic, P.; Sauerberg, M. Life Expectancy: Frequently Used, but Hardly Understood. Gerontology 2020, 66, 95–104. [Google Scholar] [CrossRef]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [Green Version]
- Crimmins, E.M. Recent trends and increasing differences in life expectancy present opportunities for multidisciplinary research on aging. Nat. Aging 2021, 1, 12–13. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Kurchashova, S.Y.; Kireev, I. Are There Common Mechanisms Between the Hutchinson–Gilford Progeria Syndrome and Natural Aging? Front. Genet. 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orioli, D.; Dellambra, E. Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Toda, I.M.; Maté, I.; Vida, C.; Cruces, J.; De La Fuente, M. Immune function parameters as markers of biological age and predictors of longevity. Aging 2016, 8, 3110–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, F.C.; Smoke, M.E. The measurement of biological age. Exp. Aging Res. 1980, 6, 497–522. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, W.C.; Scherbov, S. Measuring the Speed of Aging across Population Subgroups. PLoS ONE 2014, 9, e96289. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, M.; Tomas, J.M.; Calatayud, P. Contributions of Psychosocial Factors and Physical Activity to Successful Aging. Span. J. Psychol. 2018, 21, E26. [Google Scholar] [CrossRef]
- Gadecka, A.; Bielak-Zmijewska, A. Slowing Down Ageing: The Role of Nutrients and Microbiota in Modulation of the Epigenome. Nutrients 2019, 11, 1251. [Google Scholar] [CrossRef] [Green Version]
- Ahadi, S.; Zhou, W.; Rose, S.M.S.-F.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Kubben, N.; Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 2017, 18, 595–609. [Google Scholar] [CrossRef]
- Brietzke, E.; Cerqueira, R.O.; Soares, C.N.; Kapczinski, F. Is bipolar disorder associated with premature aging? Trends Psychiatry Psychother. 2019, 41, 315–317. [Google Scholar] [CrossRef] [Green Version]
- Ness, K.K.; Kirkland, J.L.; Gramatges, M.M.; Wang, Z.; Kundu, M.; McCastlain, K.; Li-Harms, X.; Zhang, J.; Tchkonia, T.; Pluijm, S.M.F.; et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Rebelo-Marques, A.; Lages, A.D.S.; Andrade, R.; Ribeiro, C.F.; Mota-Pinto, A.; Carrilho, F.; Espregueira-Mendes, J. Aging Hallmarks: The Benefits of Physical Exercise. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Willcox, B.J.; Donlon, T.A. Genetic and epigenetic regulation of human aging and longevity. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1718–1744. [Google Scholar] [CrossRef]
- Juckett, D.A. What determines age-related disease: Do we know all the right questions? AGE 2009, 32, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin. Chem. 2019, 65, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noels, H.; Jankowski, J. Editorial on the Special Issue “Comorbidities in Chronic Kidney Disease”. Toxins 2020, 12, 384. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-Y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Buendía, P.; Merino, A.; Soriano, S.; Esquivias, E.; Martín-Malo, A.; Aljama, P.; Ramírez, R. Cellular senescence determines endothelial cell damage induced by uremia. Exp. Gerontol. 2013, 48, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Rutenberg, A.D.; Farrell, S.; Rockwood, K. Aging, frailty and complex networks. Biogerontology 2017, 18, 433–446. [Google Scholar] [CrossRef]
- Vaupel, J.; Manton, K.G.; Stallard, E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 1979, 16, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Kin, S.K.; Yamada, S.; Akishita, M.; Ogawa, S. Sex-related differences in the association between frailty and dietary consumption in Japanese older people: A cross-sectional study. BMC Geriatr. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, J.A.; Cardoso, R.R.; Durães, M.S.; Guedes, M.C.A.; Santos, F.L.; Da Costa, F.M.; Caldeira, A.P. Frailty in the elderly: Prevalence and associated factors. Rev. Bras. de Enferm. 2017, 70, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004, 59, M255–M263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; De Sequera, P.; Ramírez, R. Mechanisms of Cardiovascular Disorders in Patients With Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of Deficits as a Proxy Measure of Aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, N. Frailty as cardiovascular risk factor (and vice versa). In Frailty and Cardiovascular Diseases. Advances in Experimental Medicine and Biology, 1st ed.; Veronese, N., Ed.; Springer AG: Cham, Switzerland, 2020; Volume 1216. [Google Scholar] [CrossRef]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef]
- Chowdhury, R.; Peel, N.; Krosch, M.; Hubbard, R. Frailty and chronic kidney disease: A systematic review. Arch. Gerontol. Geriatr. 2017, 68, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.D.; Newman, A.B.; Hirsch, C.; Gottdiener, J.S.; Seeman, T.E.; Tracy, R.P.; Kop, W.J.; Burke, G.L.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Saliba, D.; Elliott, M.; Rubenstein, L.; Solomon, D.H.; Young, R.T.; Kamberg, C.J.; Roth, R.C.; MacLean, C.H.; Shekelle, P.G.; Sloss, E.M.; et al. The Vulnerable Elders Survey: A Tool for Identifying Vulnerable Older People in the Community. J. Am. Geriatr. Soc. 2001, 49, 1691–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.; Murthy, S.; Tainter, C.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.; et al. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, P.W.; Cron, D.C.; Terjimanian, M.N.; Wang, S.C.; Englesbe, M.; Waits, S.A. Sarcopenia and failure to rescue following liver transplantation. Clin. Transplant. 2015, 29, 1076–1080. [Google Scholar] [CrossRef]
- Jones, D.; Song, X.; Mitnitski, A.; Rockwood, K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin. Exp. Res. 2005, 17, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Rockwood, M.R.H.; Mitnitski, A. Physiological Redundancy in Older Adults in Relation to the Change with Age in the Slope of a Frailty Index. J. Am. Geriatr. Soc. 2010, 58, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef] [Green Version]
- Gobbens, R.J.; van Assen, M.A.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. The Tilburg Frailty Indicator: Psychometric Properties. J. Am. Med. Dir. Assoc. 2010, 11, 344–355. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Webster, A.C.; Nagler, E.V.; Morton, R.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Glassock, R.J.; Warnock, D.G.; Delanaye, P. The global burden of chronic kidney disease: Estimates, variability and pitfalls. Nat. Rev. Nephrol. 2017, 13, 104–114. [Google Scholar] [CrossRef]
- Lv, J.-C.; Zhang, L.-X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Okusa, M.D. Effects of Aging on Renal Function and Regenerative Capacity. Nephron Clin. Pract. 2014, 127, 15–20. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Dukas, L.; Schacht, E.; Stähelin, H.B. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 mL/min is a risk factor for falls and fractures. Osteoporos. Int. 2005, 16, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delanaye, P.; Jager, K.J.; Bökenkamp, A.; Christensson, A.; Dubourg, L.; Eriksen, B.O.; Gaillard, F.; Gambaro, G.; van der Giet, M.; Glassock, R.J.; et al. CKD: A Call for an Age-Adapted Definition. J. Am. Soc. Nephrol. 2019, 30, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Tzunoda, K.; Abe, K.; Goto, T.; Yasujima, M.; Sato, M.; Omata, K.; Seino, M.; Yoshinaga, K. Effect of Age on the Renin-Angiotensin-Aldosterone System in Normal Subjects: Simultaneous Measurement of Active and Inactive Renin, Renin Substrate, and Aldosterone in Plasma. J. Clin. Endocrinol. Metab. 1986, 62, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.F.; Kennefick, T.M.; Ingelfinger, J.R.; Vora, J.P.; Anderson, S. Down-regulation of the intrarenal renin-angiotensin system in the aging rat. J. Am. Soc. Nephrol. 1995, 5, 1573–1580. [Google Scholar] [CrossRef]
- Zhou, X.J.; Saxena, R.; Liu, Z.; Vaziri, N.D.; Silva, F.G. Renal senescence in 2008: Progress and challenges. Int. Urol. Nephrol. 2008, 40, 823–839. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-O, M.; Moe, O.W. Klotho Deficiency Causes Vascular Calcification in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, B.; Izquierdo, M.C.; Valiño-Rivas, L.; Nastou, D.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Albumin downregulates Klotho in tubular cells. Nephrol. Dial. Transplant. 2018, 33, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Lei, H.; Wang, X.; Wang, Y.; Sonntag, W.; Sun, Z. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. AGE 2011, 33, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Suárez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The Inflammatory Cytokines TWEAK and TNFα Reduce Renal Klotho Expression through NFκB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef] [Green Version]
- White, W.E. Aging and uremia: Is there cellular and molecular crossover? World J. Nephrol. 2015, 4, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Huang, J.-C.; Su, H.-M.; Chiu, Y.-W.; Chang, J.-M.; Hwang, S.-J.; Chen, H.-C. Prognostic Cardiovascular Markers in Chronic Kidney Disease. Kidney Blood Press. Res. 2018, 43, 1388–1407. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Mitesh, S.; Gkogkou, A.; Geladari, E. Chronic Kidney Disease and Cardiovascular Disease: Is there Any Relationship? Curr. Cardiol. Rev. 2019, 15, 55–63. [Google Scholar] [CrossRef]
- Merino, A.; Nogueras, S.; Buendia, P.; Ojeda, R.; Carracedo, J.; Ramirez-Chamond, R.; Martín-Malo, A.; Aljama, P. Microinflammation and Endothelial Damage in Hemodialysis. Contrib. Nephrol. 2008, 161, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S. Pathogenesis of endothelial cell dysfunction in chronic kidney disease: A retrospective and what the future may hold. Kidney Res. Clin. Pr. 2015, 34, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, A.; Buendia, P.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Carracedo, J. Senescent CD14+CD16+Monocytes Exhibit Proinflammatory and Proatherosclerotic Activity. J. Immunol. 2011, 186, 1809–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellam, T.J.; Chico, T. Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 2012, 220, 310–318. [Google Scholar] [CrossRef]
- Six, I.; Maizel, J.; Barreto, F.C.; Rangrez, A.; Dupont, S.; Slama, M.; Tribouilloy, C.; Choukroun, G.; Mazière, J.C.; Bode-Boeger, S.; et al. Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc. Res. 2012, 96, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, G.S.; König, M.; Stock, C.; Wiesinger, A.; Hillebrand, U.; Reiermann, S.; Reuter, S.; Amler, S.; Köhler, G.; Buck, F.; et al. High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int. 2013, 83, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Shuto, E.; Taketani, Y.; Tanaka, R.; Harada, N.; Isshiki, M.; Sato, M.; Nashiki, K.; Amo, K.; Yamamoto, H.; Higashi, Y.; et al. Dietary Phosphorus Acutely Impairs Endothelial Function. J. Am. Soc. Nephrol. 2009, 20, 1504–1512. [Google Scholar] [CrossRef] [Green Version]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef] [Green Version]
- Cuenca, M.V.; Hordijk, P.L.; Vervloet, M.G. Most exposed: The endothelium in chronic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Piroddi, M.; Annetti, C.; Aisa, C.; Floridi, E.; Floridi, A. Oxidative Stress and Reactive Oxygen Species. Contrib. Nephrol. 2005, 149, 240–260. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-H.; Okada, S.; Ly, S.; Jandeleit-Dahm, K.; Barit, D.; Namikoshi, T.; Sharma, K. Role of Nox2 in diabetic kidney disease. Am. J. Physiol. Physiol. 2013, 304, F840–F848. [Google Scholar] [CrossRef]
- Simone, S.; Rascio, F.; Castellano, G.; Divella, C.; Chieti, A.; Ditonno, P.; Battaglia, M.; Crovace, A.; Staffieri, F.; Oortwijn, B.; et al. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free. Radic. Biol. Med. 2014, 74, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Annuk, M.; Zilmer, M.; Lind, L.; Linde, T.; Fellström, B. Oxidative Stress and Endothelial Function in Chronic Renal Failure. J. Am. Soc. Nephrol. 2001, 12, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative Stress Is Progressively Enhanced With Advancing Stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, E.; Wautier, M.-P.; Wautier, J.-L.; Boval, B.; Panis, Y.; Wernert, N.; Danze, P.-M.; Dequiedt, P. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 2002, 61, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Batista Júnior, M.L.; Lopes, R.D.; Seelaender, M.C.L.; Lopes, A.C. Anti-inflammatory effect of physical training in heart failure: Role of TNF-alpha and IL-10. Arq. Bras. Cardiol. 2009, 93, 643–651. [Google Scholar]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Li, L.; Erlandsson, H.; Jaminon, A.M.G.; Qureshi, A.R.; Ripsweden, J.; Brismar, T.B.; Witasp, A.; Heimbürger, O.; Jørgensen, H.S.; et al. Functional vitamin K insufficiency, vascular calcification and mortality in advanced chronic kidney disease: A cohort study. PLoS ONE 2021, 16, e0247623. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Fuente, M.; Miquel, J. An Update of the Oxidation-Inflammation Theory of Aging: The Involvement of the Immune System in Oxi-Inflamm-Aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Sea, M.M.-M.; Tang, N.; Sanderson, J.E.; Lui, S.-F.; Li, P.K.-T.; Woo, J. Resting Energy Expenditure and Subsequent Mortality Risk in Peritoneal Dialysis Patients. J. Am. Soc. Nephrol. 2004, 15, 3134–3143. [Google Scholar] [CrossRef] [Green Version]
- Suliman, M.E.; Heimbürger, O.; Barany, P.; Anderstam, B.; Pecoits-Filho, R.; Ayala, E.R.; Qureshi, A.R.T.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Plasma Pentosidine Is Associated with Inflammation and Malnutrition in End-Stage Renal Disease Patients Starting on Dialysis Therapy. J. Am. Soc. Nephrol. 2003, 14, 1614–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, E.; Bajo, M.-A.; Carrero, J.J.; Lindholm, B.; Grande, C.; Sánchez-Villanueva, R.; Del Peso, G.; Díaz-Almirón, M.; Iglesias, P.; Díez, J.J.; et al. An Increase of Plasma Advanced Oxidation Protein Products Levels Is Associated with Cardiovascular Risk in Incident Peritoneal Dialysis Patients: A Pilot Study. Oxidative Med. Cell. Longev. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memoli, B.; Minutolo, R.; Bisesti, V.; Postiglione, L.; Conti, A.; Marzano, L.; Capuano, A.; Andreucci, M.; Balletta, M.M.; Guida, B.; et al. Changes of serum albumin and C-reactive protein are related to changes of interleukin-6 release by peripheral blood mononuclear cells in hemodialysis patients treated with different membranes. Am. J. Kidney Dis. 2002, 39, 266–273. [Google Scholar] [CrossRef]
- Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Temmar, M.; Lemke, H.-D.; Tribouilloy, C.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010, 77, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rizza, G.M.; Taccola, D.; Paoletti, S.; Mantuano, E.; Migliori, M.; Frangioni, S.; Filippi, C.; Carpi, A. C-reactive protein in patients on chronic hemodialysis with different techniques and different membranes. Biomed. Pharmacother. 2006, 60, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Van Tellingen, A.; Grooteman, M.P.; Schoorl, M.; Bartels, P.C.; Schoorl, M.; Van Der Ploeg, T.; Ter Wee, P.M.; Nubé, M.J. Intercurrent clinical events are predictive of plasma C-reactive protein levels in hemodialysis patients. Kidney Int. 2002, 62, 632–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, R.; Carracedo, J.; Berdud, I.; Carretero, D.; Merino, A.; Rodriguez, M.; Tetta, C.; Martin-Malo, A.; Aljama, P. Microinflammation in hemodialysis is related to a preactivated subset of monocytes. Hemodial. Int. 2006, 10, S24–S27. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Soveri, I.; Hilborn, J.; Fellström, B.; Nilsson, B. Cardiovascular disease in haemodialysis: Role of the intravascular innate immune system. Nat. Rev. Nephrol. 2017, 13, 285–296. [Google Scholar] [CrossRef]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; De Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-Reactive Protein and Prediction of 1-Year Mortality in Prevalent Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2452–2461. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, B.; Alexander, D.; Janessa, A.; Häring, H.-U.; Lang, F.; Risler, T. Acute effects of hemodialysis on cytokine transcription profiles: Evidence for C-reactive protein-dependency of mediator induction. Kidney Int. 2006, 70, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Feroze, U.; Molnar, M.Z.; Dukkipati, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Insights Into Nutritional and Inflammatory Aspects of Low Parathyroid Hormone in Dialysis Patients. J. Ren. Nutr. 2011, 21, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Lin, Y.-C.; Hsu, C.-Y.; Kao, C.-C.; Chang, F.-C.; Chen, T.-W.; Chen, H.-H.; Hsu, C.-C.; Wu, M.-S.; Taiwan Society of Nephrology. Effect Modifying Role of Serum Calcium on Mortality-Predictability of PTH and Alkaline Phosphatase in Hemodialysis Patients: An Investigation Using Data from the Taiwan Renal Registry Data System from 2005 to 2012. PLoS ONE 2015, 10, e0129737. [Google Scholar] [CrossRef] [Green Version]
- Taraz, M.; Taraz, S.; Dashti-Khavidaki, S. Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: A review of literature. Hemodial. Int. 2014, 19, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoo, T.-H.; Hwang, Y.; Lee, G.H.; Kim, B.; Jang, J.; Yu, H.T.; Kim, M.C.; Cho, J.-Y.; Lee, C.J.; et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Hörl, W.H.; Cohen, J.J.; Harrington, J.T.; Madias, N.E.; Zusman, C.J. Atherosclerosis and uremic retention solutes. Kidney Int. 2004, 66, 1719–1731. [Google Scholar] [CrossRef] [Green Version]
- Panuccio, V.; Enia, G.; Tripepi, R.; Aliotta, R.; Mallamaci, F.; Tripepi, G.; Zoccali, C. Pro-inflammatory cytokines and bone fractures in CKD patients. An exploratory single centre study. BMC Nephrol. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neirynck, N.; Glorieux, G.; Schepers, E.; Verbeke, F.; Vanholder, R. Soluble Tumor Necrosis Factor Receptor 1 and 2 Predict Outcomes in Advanced Chronic Kidney Disease: A Prospective Cohort Study. PLoS ONE 2015, 10, e0122073. [Google Scholar] [CrossRef] [Green Version]
- Parikova, A.; Hruba, P.; Krediet, R.; Krejcik, Z.; Stranecky, V.; Striz, I.; Viklicky, O. Long-Term Peritoneal Dialysis Treatment Provokes Activation of Genes Related to Adaptive Immunity. Physiol. Res. 2019, 775–783. [Google Scholar] [CrossRef]
- Pérez-García, R.; Ortiz, P.D.S.; Albalate, M.; Carretero, M.P.; Ortega, M.; Caro, M.C.R.; Chamond, R.R.; Arroyo, R.A. Citrate dialysate does not induce oxidative stress or inflammation in vitro as compared to acetate dialysate. Nefrologia 2017, 37, 630–637. [Google Scholar] [CrossRef]
- Vida, C.; Carracedo, J.; De Sequera, P.; Bodega, G.; Pérez, R.; Alique, M.; Ramírez, R. Increasing the Magnesium Concentration in Various Dialysate Solutions Differentially Modulates Oxidative Stress in a Human Monocyte Cell Line. Antioxidants 2020, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as mediators of intercellular communication in cancer—The emerging science of cellular ‘debris’. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J.; Yarani, R.; Roursgaard, M.; Mathiesen, E.R.; Damm, P.; Svare, J.; Mortensen, H.B.; et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes From Mothers With Type 1 Diabetes Contain Aberrant Levels of microRNAs. Front. Immunol. 2019, 10, 2543. [Google Scholar] [CrossRef]
- Carmona, A.; Guerrero, F.; Buendia, P.; Obrero, T.; Aljama, P.; Carracedo, J. Microvesicles Derived from Indoxyl Sulfate Treated Endothelial Cells Induce Endothelial Progenitor Cells Dysfunction. Front. Physiol. 2017, 8, 666. [Google Scholar] [CrossRef]
- Carracedo, J.; Ramirez, R.; Soriano, S.; De Lara, M.A.A.; Rodriguez, M.; Martin-Malo, A.; Aljama, P. Monocytes from Dialysis Patients Exhibit Characteristics of Senescent Cells: Does It Really Mean Inflammation? Contrib. Nephrol. 2005, 149, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yang, J.; Cao, W.; Sun, Y. Differential diagnosis of acute rejection and chronic cyclosporine nephropathy after rat renal transplantation by detection of endothelial microparticles (EMP). Med. Hypotheses 2010, 75, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shi, L.; Zhang, H.; Wang, C.; Gao, S.; Ma, Y.; Li, W.; Liu, J.; Wang, J.; Liu, J. Effect of alprostadil on serum level of miRNA-155 in uremic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2015, 40. [Google Scholar] [CrossRef]
- Vigorito, E.; Kohlhaas, S.; Lu, D.; Leyland, R. miR-155: An ancient regulator of the immune system. Immunol. Rev. 2013, 253, 146–157. [Google Scholar] [CrossRef]
- Li, H.; Qiu, F.; Tian, F.; Shi, X.; Gao, A.; Song, L.; Liu, J. Changes of miR-155 expression in serum of uremic patients before and after treatment and risk factors analysis. Exp. Ther. Med. 2020, 20, 3352–3360. [Google Scholar] [CrossRef]
- Alique, M.; Bodega, G.; Corchete, E.; García-Menéndez, E.; De Sequera, P.; Luque, R.; Rodríguez-Padrón, D.; Marqués, M.; Portolés, J.; Carracedo, J.; et al. Microvesicles from indoxyl sulfate-treated endothelial cells induce vascular calcification in vitro. Comput. Struct. Biotechnol. J. 2020, 18, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Brunet, P.; Gondouin, B.; Duval-Sabatier, A.; Dou, L.; Cerini, C.; Dignat-George, F.; Jourde-Chiche, N.; Argiles, A.; Burtey, S. Does Uremia Cause Vascular Dysfunction? Kidney Blood Press. Res. 2011, 34, 284–290. [Google Scholar] [CrossRef]
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729. [Google Scholar] [CrossRef]
- Tchkonia, T.; Zhu, Y.; Van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.-J.; Cai, G.-Y.; Chen, X.-M. Cellular senescence, senescence-associated secretory phenotype, and chronic kidney disease. Oncotarget 2017, 8, 64520–64533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alique, M.; Ramírez-Carracedo, R.; Bodega, G.; Carracedo, J.; Ramírez, R. Senescent Microvesicles: A Novel Advance in Molecular Mechanisms of Atherosclerotic Calcification. Int. J. Mol. Sci. 2018, 19, 2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano, G.; Franzin, R.; Sallustio, F.; Stasi, A.; Banelli, B.; Romani, M.; De Palma, G.; Lucarelli, G.; Divella, C.; Battaglia, M.; et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging 2019, 11, 4382–4406. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, F.; Bhandari, S. Oxidative Stress and Cardiovascular Complications in Chronic Kidney Disease, the Impact of Anaemia. Pharmaceuticals 2018, 11, 103. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figuer, A.; Bodega, G.; Tato, P.; Valera, G.; Serroukh, N.; Ceprian, N.; de Sequera, P.; Morales, E.; Carracedo, J.; Ramírez, R.; et al. Premature Aging in Chronic Kidney Disease: The Outcome of Persistent Inflammation beyond the Bounds. Int. J. Environ. Res. Public Health 2021, 18, 8044. https://doi.org/10.3390/ijerph18158044
Figuer A, Bodega G, Tato P, Valera G, Serroukh N, Ceprian N, de Sequera P, Morales E, Carracedo J, Ramírez R, et al. Premature Aging in Chronic Kidney Disease: The Outcome of Persistent Inflammation beyond the Bounds. International Journal of Environmental Research and Public Health. 2021; 18(15):8044. https://doi.org/10.3390/ijerph18158044
Chicago/Turabian StyleFiguer, Andrea, Guillermo Bodega, Patricia Tato, Gemma Valera, Nadia Serroukh, Noemi Ceprian, Patricia de Sequera, Enrique Morales, Julia Carracedo, Rafael Ramírez, and et al. 2021. "Premature Aging in Chronic Kidney Disease: The Outcome of Persistent Inflammation beyond the Bounds" International Journal of Environmental Research and Public Health 18, no. 15: 8044. https://doi.org/10.3390/ijerph18158044
APA StyleFiguer, A., Bodega, G., Tato, P., Valera, G., Serroukh, N., Ceprian, N., de Sequera, P., Morales, E., Carracedo, J., Ramírez, R., & Alique, M. (2021). Premature Aging in Chronic Kidney Disease: The Outcome of Persistent Inflammation beyond the Bounds. International Journal of Environmental Research and Public Health, 18(15), 8044. https://doi.org/10.3390/ijerph18158044