Key Factors for Improving the Carcinogenic Risk Assessment of PAH Inhalation Exposure by Monte Carlo Simulation
Abstract
:1. Introduction
2. Materials and Methods
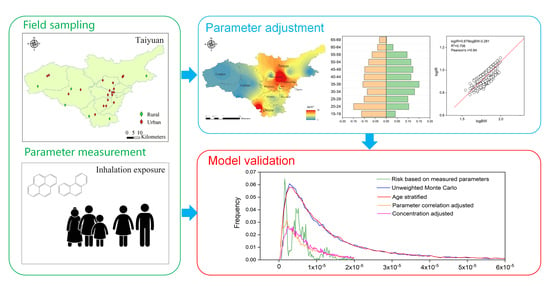
2.1. Research Area and Participants
2.2. Sampling and Analytical Methods
2.3. Risk Characterization
2.4. Monte Carlo Simulation
3. Results and Discussion
3.1. Individual Actual Risks Based on Measured Parameters
3.2. Population Risk Based on Monte Carlo Simulation
3.2.1. Parameter Distribution Estimation
3.2.2. Comparison of Risk Distribution between the Two Methods
3.2.3. Parameter Sensitivity Analysis
3.3. Key Factors for Improving the Monte Carlo Simulation
3.4. Improved Models
3.4.1. Concentration-Adjusted Monte Carlo
3.4.2. Age-Stratified Monte Carlo
3.4.3. Parameter-Correlation-Adjusted Monte Carlo
3.5. Comparison of Improved Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Xu, X.; Georgopoulos, P.G. A Bayesian population PBPK model for multiroute chloroform exposure. J. Expo. Sci. Environ. Epidemiol. 2009, 20, 326–341. [Google Scholar] [CrossRef] [Green Version]
- Lester, R.R.; Green, L.C.; Linkov, I. Site-specific applications of probabilistic health risk assessment: Review of the literature since 2000. Risk Anal. 2007, 27, 635–658. [Google Scholar] [CrossRef] [PubMed]
- Glorennec, P.; Bemrah, N.; Tard, A.; Robin, A.; Le Bot, B.; Bard, D. Probabilistic modeling of young children’s overall lead exposure in France: Integrated approach for various exposure media. Environ. Int. 2007, 33, 937–945. [Google Scholar] [CrossRef]
- Mishra, H.; Singh, J.; Karmakar, S.; Kumar, R. An integrated approach for modeling uncertainty in human health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 56053–56068. [Google Scholar] [CrossRef] [PubMed]
- Burmaster, D.E.; Anderson, P.D. Principles of Good Practice for the Use of Monte Carlo Techniques in Human Health and Ecological Risk Assessments. Risk Anal. 1994, 14, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Burmaster, D.E.; Crouch, E.A. Monte Carlo Techniques for Quantitative Uncertainty Analysis in Public Health Risk Assessments. Risk Anal. 1992, 12, 53–63. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Duan, L.; Bekele, D.; Naidu, R. Uncertainties in human health risk assessment of environmental contaminants: A review and perspective. Environ. Int. 2015, 85, 120–132. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Sun, J.; Li, X.; Geng, X.; Zhao, M.; Sun, T.; Fan, Z. Health risk assessment of heavy metal(loid)s in park soils of the largest megacity in China by using Monte Carlo simulation coupled with Positive matrix factorization model. J. Hazard. Mater. 2021, 415, 125629. [Google Scholar] [CrossRef]
- Sanaei, F.; Amin, M.M.; Alavijeh, Z.P.; Esfahani, R.A.; Sadeghi, M.; Bandarrig, N.S.; Fatehizadeh, A.; Taheri, E.; Re-zakazemi, M. Health risk assessment of potentially toxic elements intake via food crops consumption: Monte Carlo simu-lation-based probabilistic and heavy metal pollution index. Environ. Sci. Pollut. Res. 2021, 28, 1479–1490. [Google Scholar] [CrossRef]
- Dai, H.; Jing, S.; Wang, H.; Ma, Y.; Li, L.; Song, W.; Kan, H. VOC characteristics and inhalation health risks in newly renovated residences in Shanghai, China. Sci. Total Environ. 2017, 577, 73–83. [Google Scholar] [CrossRef]
- Huang, D.; Liu, M.; Zhang, J.; Wang, Y. Research on risk assessment based on Monte Carlo simulation and dose-response multistage model. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; Volume 3, pp. 1245–1250. [Google Scholar]
- Simon, S.L.; Hoffman, F.O.; Hofer, E. The two-dimensional Monte Carlo: A new methodologic paradigm for dose reconstruction for epidemiological studies. Radiat. Res. 2015, 183, 27–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-C.; Niu, Z.-G.; Zhang, Y. Health risk assessment of inhalation exposure of irrigation workers and the public to trihalomethanes from reclaimed water in landscape irrigation in Tianjin, North China. J. Hazard. Mater. 2013, 262, 179–188. [Google Scholar] [CrossRef]
- Luo, K.; Zeng, Y.; Li, M.; Man, Y.; Zeng, L.; Zhang, Q.; Luo, J.; Kang, Y. Inhalation bioacessibility and absorption of polycyclic aromatic hydrocarbons (PAHs) in indoor PM2.5 and its implication in risk assessment. Sci. Total Environ. 2021, 774, 145770. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; He, W.; Liu, W.; Kong, X.; Xu, F.; Giesy, J.P. Tissue distribution, bioaccumulation, and carcinogenic risk of polycyclic aromatic hydrocarbons in aquatic organisms from Lake Chaohu, China. Sci. Total Environ. 2020, 749, 141577. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, L.; Ren, A.; Yuan, Y.; Liu, J.; Li, Z.; Zhang, L.; Yi, D.; Wang, L.-L.; Zhang, Y.; et al. Levels of Polycyclic Aromatic Hydrocarbons in Maternal Serum and Risk of Neural Tube Defects in Offspring. Environ. Sci. Technol. 2015, 49, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liang, Y.; Zhao, B.; Wang, Y.; Xing, F.; Qin, L. Polycyclic aromatic hydrocarbon (PAHs) geographical distribu-tion in China and their source, risk assessment analysis. Environ. Pollut. 2019, 251, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R.T. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: Roads as sources and sinks. Environ. Sci. Technol. 1993, 27, 1892–1904. [Google Scholar] [CrossRef]
- Da Silva, F.C.; Felipe, M.; de Castro, D.E.F.; Araujo, S.C.D.; Sisenando, H.C.N.; de Medeiros, S.R.B. A look beyond the priority: A systematic review of the genotoxic, mutagenic, and carcinogenic endpoints of non-priority PAHs *. Environ. Pollut. 2021, 278, 116838. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Kong, X.-Z.; He, W.; He, Q.-S.; Liu, W.-X.; Xu, F.-L. Dustfall-bound polycyclic aromatic hydrocarbons (PAHs) over the fifth largest Chinese lake: Residual levels, source apportionment, and correlations with suspended particulate matter (SPM)-bound PAHs in water. Environ. Sci. Pollut. Res. 2021, 28, 55388–55400. [Google Scholar] [CrossRef]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997, 8, 444–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Kuang, D.; Zhang, W.; Deng, Q.; Zhang, X.; Huang, K.; Guan, L.; Hu, D.; Wu, T.; Guo, H. Dose-Response Relationships of Polycyclic Aromatic Hydrocarbons Exposure and Oxidative Damage to DNA and Lipid in Coke Oven Workers. Environ. Sci. Technol. 2013, 47, 7446–7456. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.G.; Doust, E.; Cherrie, J.W.; Hurley, J.F. Lung cancer mortality and exposure to polycyclic aromatic hydrocar-bons in British coke oven workers. BMC Public Health 2013, 13, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Duan, X.; Qin, N.; Li, J.; Tian, J.; Zhong, Y.; Chen, L.; Fan, R.; Yu, Y.; Wu, G.; et al. Internal biomarkers and external estimation of exposure to polycyclic aromatic hydrocarbons and their relationships with cancer mortality in a high cancer incidence area. Sci. Total Environ. 2019, 688, 742–750. [Google Scholar] [CrossRef]
- Zhen, Z.; Yin, Y.; Chen, K.; Zhen, X.; Zhang, X.; Jiang, H.; Wang, H.; Kuang, X.; Cui, Y.; Dai, M.; et al. Concentration and atmospheric transport of PM2.5-bound polycyclic aromatic hydrocarbons at Mount Tai, China. Sci. Total Environ. 2021, 786, 147513. [Google Scholar] [CrossRef]
- Zhou, S.; Hwang, B.; Lakey, P.; Zuend, A.; Abbatt, J.P.D.; Shiraiwa, M. Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations. Proc. Natl. Acad. Sci. USA 2019, 116, 11658–11663. [Google Scholar] [CrossRef] [Green Version]
- Petit, P.; Maître, A.; Persoons, R.; Bicout, D.J. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ. Int. 2019, 124, 109–120. [Google Scholar] [CrossRef]
- Li, R.; Cheng, M.; Cui, Y.; He, Q.; Guo, X.; Chen, L.; Wang, X. Distribution of the Soil PAHs and Health Risk Influenced by Coal Usage Processes in Taiyuan City, Northern China. Int. J. Environ. Res. Public Health 2020, 17, 6319. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, J.; Wang, Z.; Zhao, L.; Zhang, H.; Li, M. Determination and source identification of priority polycyclic aromatic hydrocarbons in PM2.5 in Taiyuan, China. Atmos. Res. 2016, 178–179, 401–414. [Google Scholar] [CrossRef]
- Yan, Y.; He, Q.; Guo, L.; Li, H.; Zhang, H.; Shao, M.; Wang, Y. Source apportionment and toxicity of atmospheric poly-cyclic aromatic hydrocarbons by PMF: Quantifying the influence of coal usage in Taiyuan, China. Atmos. Res. 2017, 193, 50–59. [Google Scholar] [CrossRef]
- Liu, Y.W.; Qin, N.; Liang, W.G.; Chen, X.; Hou, R.; Kang, Y.J.; Guo, Q.; Cao, S.Z.; Duan, X.L. Polycycl. Aromatic Hydrocarbon Exposure of Children in Typical Household Coal Combustion Environments: Seasonal Variations, Sources, and Carcinogenic Risks. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef]
- China Statistical Publishing House. Shanxi Statistical Yearbook; China Statistics Press: Beijing, China, 2009.
- Shanxi Province Bureau of Statistics. Tabulation on the 2010 Population Census of Shanxi Province; China Statistics Press: Beijing, China, 2012.
- Xia, Z.; Duan, X.; Tao, S.; Qiu, W.; Liu, D.; Wang, Y.; Wei, S.; Wang, B.; Jiang, Q.; Lu, B.; et al. Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ. Pollut. 2013, 173, 150–156. [Google Scholar] [CrossRef]
- Duan, X.; Wang, B.; Zhao, X.; Shen, G.; Xia, Z.; Huang, N.; Jiang, Q.; Lu, B.; Xu, D.; Fang, J.; et al. Personal inhalation exposure to polycyclic aromatic hydrocarbons in urban and rural residents in a typical northern city in China. Indoor Air 2014, 24, 464–473. [Google Scholar] [CrossRef]
- USEPA. Guidelines for human exposure assessment. In Risk Assessment Forum; USEPA: Washington, DC, USA, 2019. [Google Scholar]
- Chen, S.-C.; Liao, C.-M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.M.; Chio, C.P.; Chen, W.Y.; Ju, Y.R.; Li, W.H.; Cheng, Y.H.; Liao, V.H.C.; Chen, S.C.; Ling, M.P. Lung cancer risk in relation to traffic-related nano/ultrafine particle-bound PAHs exposure: A preliminary probabilistic assessment. J. Hazard. Mater. 2011, 190, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Duan, X. Exposure Factors Handbook of Chinese Population; Ministry of Environmental Protection of the People’s Republic of China; China Environmental Science Press: Beijing, China, 2014.
- Culle, A.; Frey, H. Probabilistic Techniques in Exposure Assessment: A Handbook for Dealing with Variability and Uncertainty in Models and Inputs; Plenum Press: New York, NY, USA, 1999. [Google Scholar]
- Zhang, M.; Wang, J.; Zhao, Q.; Mishra, V.; Fan, J.; Sun, Y. Polycyclic aromatic hydrocarbons (PAHs) and esophageal carcinoma in Handan-Xingtai district, North China: A preliminary study based on cancer risk assessment. Environ. Monit. Assess. 2020, 192, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Fu, N.; Zhang, S.; Du, W.; Chen, Y.; Wang, Z.; Qi, M.; Wang, W.; Zhong, Q.; et al. Inhalation exposure to size-segregated fine particles and particulate PAHs for the population burning biomass fuels in the Eastern Tibetan Plateau area. Ecotoxicol. Environ. Saf. 2021, 211, 111959. [Google Scholar] [CrossRef]
- Bi, C.; Chen, Y.; Zhao, Z.; Li, Q.; Zhou, Q.; Ye, Z.; Ge, X. Characteristics, sources and health risks of toxic species (PCDD/Fs, PAHs and heavy metals) in PM2.5 during fall and winter in an industrial area. Chemosphere 2020, 238, 124620. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Kong, X.Z.; Liu, W.X.; He, Q.S.; Yang, B.; Ouyang, H.L.; Wang, Q.M.; Xu, F.L. Atmospheric parti-tioning and the air-water exchange of polycyclic aromatic hydrocarbons in a large shallow Chinese lake (Lake Chaohu). Chemosphere 2013, 93, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Duan, X.; Qin, N.; Lv, J.; Wu, G.; Wei, F. Health risk from dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in a typical high cancer incidence area in southwest China. Sci. Total Environ. 2019, 649, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, M.; Yunesian, M.; Nabizadeh, R.; Yaghmaeian, K.; Ahmadkhaniha, R.; Rastkari, N.; Parmy, S.; Faridi, S.; Rafiee, A.; Naddafi, K. Characterization and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban atmospheric Particulate of Tehran, Iran. Environ. Sci. Pollut. Res. 2016, 23, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, I.C.T.; Lagoy, P.K. Toxic equaivalency factors (TEFs) for Polycyclic Aromatic-Hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Layton, D.W. Metabolically consistent breathing rates for use in dose assessments. Health Phys. 1993, 64, 23–36. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Exposure Factors Handbook; USEPA: Washington, DC, USA, 2011.







| N | Height (cm) | Body Weight (kg) | Inhalation Rate a (L/min) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| SEX | |||||||
| Male | 1305 | 171 | 5.41 | 67.7 | 8.8 | 14.04 | 0.73 |
| Female | 1435 | 160 | 5.80 | 56.9 | 10.1 | 11.07 | 1.17 |
| AREA | |||||||
| Urban | 1382 | 166 | 7.53 | 63.0 | 11.0 | 12.51 | 1.83 |
| Rural | 1358 | 164 | 7.84 | 60.6 | 10.6 | 12.30 | 1.71 |
| Age group | |||||||
| 16 ~< 25 years | 450 | 166 | 8.06 | 57.4 | 10.7 | 12.17 | 2.03 |
| 26 ~< 35 years | 626 | 166 | 7.48 | 61.3 | 10.8 | 12.48 | 1.87 |
| 36 ~< 45 years | 737 | 164 | 7.34 | 62.9 | 10.5 | 12.47 | 1.67 |
| 46 ~< 55 years | 618 | 165 | 7.67 | 63.7 | 10.8 | 12.56 | 1.64 |
| 56 ~< 65 years | 303 | 163 | 8.31 | 63.0 | 11.0 | 12.14 | 1.66 |
| 66 ~< 75 years | 6 | 171 | 10.89 | 70.5 | 12.0 | 11.68 | 1.60 |
| Age Subgroup (Years) | BW (kg) | IR (m3·day−1) | ||||
|---|---|---|---|---|---|---|
| a | b | R2 | a | b | R2 | |
| 18–44 | 4.169 | 0.225 | 0.991 | 2.823 | 0.175 | 0.944 |
| 45–59 | 4.192 | 0.204 | 0.991 | 2.816 | 0.150 | 0.986 |
| 60–70 | 4.142 | 0.216 | 0.993 | 2.660 | 0.157 | 0.988 |
| Whole population | 4.169 | 0.218 | 0.992 | 2.788 | 0.195 | 0.995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, N.; Tuerxunbieke, A.; Wang, Q.; Chen, X.; Hou, R.; Xu, X.; Liu, Y.; Xu, D.; Tao, S.; Duan, X. Key Factors for Improving the Carcinogenic Risk Assessment of PAH Inhalation Exposure by Monte Carlo Simulation. Int. J. Environ. Res. Public Health 2021, 18, 11106. https://doi.org/10.3390/ijerph182111106
Qin N, Tuerxunbieke A, Wang Q, Chen X, Hou R, Xu X, Liu Y, Xu D, Tao S, Duan X. Key Factors for Improving the Carcinogenic Risk Assessment of PAH Inhalation Exposure by Monte Carlo Simulation. International Journal of Environmental Research and Public Health. 2021; 18(21):11106. https://doi.org/10.3390/ijerph182111106
Chicago/Turabian StyleQin, Ning, Ayibota Tuerxunbieke, Qin Wang, Xing Chen, Rong Hou, Xiangyu Xu, Yunwei Liu, Dongqun Xu, Shu Tao, and Xiaoli Duan. 2021. "Key Factors for Improving the Carcinogenic Risk Assessment of PAH Inhalation Exposure by Monte Carlo Simulation" International Journal of Environmental Research and Public Health 18, no. 21: 11106. https://doi.org/10.3390/ijerph182111106
APA StyleQin, N., Tuerxunbieke, A., Wang, Q., Chen, X., Hou, R., Xu, X., Liu, Y., Xu, D., Tao, S., & Duan, X. (2021). Key Factors for Improving the Carcinogenic Risk Assessment of PAH Inhalation Exposure by Monte Carlo Simulation. International Journal of Environmental Research and Public Health, 18(21), 11106. https://doi.org/10.3390/ijerph182111106