Organophosphate Flame Retardants and Perfluoroalkyl Substances in Drinking Water Treatment Plants from Korea: Occurrence and Human Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
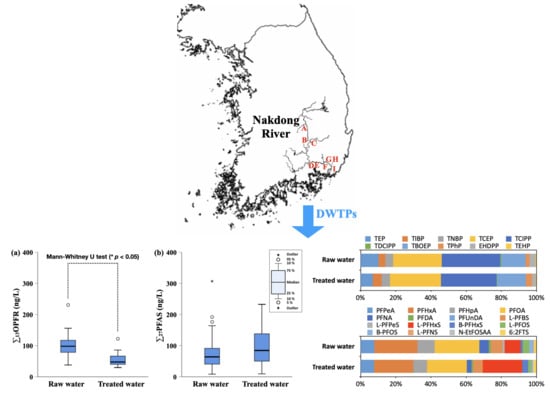
2.2. Sample Collection
2.3. Sample Preparation
2.4. Instrumental Analysis
2.5. Quality Assurance/Qaulity Control
2.6. Calculation of Estimated Daily Intake
3. Results and Discussion
3.1. Concentrations of OPFR and PFAS in Raw Water and Treated Water
3.2. Distribution and Remvoal of OPFR and PFAS in DWTPs
3.3. Exposure Assessment of OPFR and PFAS via Drinking Water Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marklund, A.; Andersson, B.; Haglund, P. Organophosphorus Flame Retardants and Plasticizers in Swedish Sewage Treatment Plants. Environ. Sci. Technol. 2005, 39, 7423–7429. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, L.T.; Buck, R.C.; Korzenoiwski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zushi, Y.; Takeda, T.; Masunaga, S. Existence of nonpoint source of perfluorinated compounds and their loads in the Tsurumi River basin, Japan. Chemosphere 2008, 71, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 6, 653. [Google Scholar] [CrossRef] [Green Version]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Egloff, C.; Crump, D.; Porter, E.; Williams, K.L.; Letcher, R.J.; Gauthier, L.T.; Kennedy, S.W. Tris(2-butoxyethyl) phosphate and triethyl phosphate alter embryonic development, hepatic mRNA expression, thyroid hormone levels, and circulating bile acid concentrations in chicken embryos. Toxicol. Appl. Pharmacol. 2014, 279, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Sturm, R.; Xie, Z.; Cai, M.; He, J.; Ebinghaus, R. Organophosphorus Flame Retardants and Plasticizers in Airborne Particles over the Northern Pacific and Indian Ocean toward the Polar Regions: Evidence for Global Occurrence. Environ. Sci. Technol. 2012, 46, 3127–3134. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Cheng, C.-G.; Wang, X.-L.; Shi, S.-H.; Wang, M.-L.; Zhao, R.-S. Preconcentration and determination of perfluoroalkyl substances (pfass) in water samples by bamboo charcoal-based solid-phase extraction prior to liquid chromatography-tandem mass spectrometry. Molecules 2018, 23, 902. [Google Scholar] [CrossRef] [Green Version]
- Alexander, B.H.; Olsen, G.W.; Burris, J.M.; Mandel, J.H.; Mandel, J.S. Mortality of employees of a perfluorooctanesulphonyl fluoride manufacturing facility. Occup. Environ. Med. 2003, 60, 722–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [Green Version]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Directive 2014/79/EU. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014L0079&from=DE (accessed on 20 June 2014).
- Government of Canada. Regulations Amending Schedule 2 to the Canada Consumer Product Safety Act (TCEP). 2014. Available online: http://www.gazette.gc.ca/rp-pr/p2/2014/2014-04-23/html/sor-dors79-eng.php (accessed on 23 April 2014).
- Washington Toxics Coalition. Governor Inslee to Sign Strongest Ban in Nation on Toxic Flame Retardants in Home Furniture, Kids’ Products. 2016. Available online: https://toxicfreefuture.org/governor-inslee-to-sign-strongest-ban-in-nation-on-toxic-flame-retardants-in-home-furniture-kids-products/ (accessed on 31 March 2016).
- European Commission. Commission Regulation (EU) 2017/1000. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R1000&from=EN (accessed on 13 June 2017).
- UNEP. Stockholm Convention on Persistent Organic Pollutants. Am. J. Int. Law 2009, 95, 692–708. [Google Scholar] [CrossRef]
- UNEP. Perfluorooctanoic acid (PFOA), its Salts and PFOA-Related Compounds. 2017. Available online: http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC13/MeetingDocuments/tabid/6024/ctl/Download/mid/17802/Default.aspx?id=113&ObjID=24612 (accessed on 16 November 2017).
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.-B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef] [PubMed]
- Noorlander, C.W.; van Leeuwen, S.P.J.; Te Biesebeek, J.D.; Mengelers, M.J.B.; Zeilmaker, M.J. Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. J. Agric. Food Chem. 2011, 59, 7496–7505. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choo, G.; Kim, H.; Oh, J.E. Evaluation of the current contamination status of PFASs and OPFRs in South Korean tap water associated with its origin. Sci. Total Environ. 2018, 634, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Eaglesham, G.; Mueller, J. Concentrations of PFOS, PFOA and other perfluorinated alkyl acids in Australian drinking water. Chemosphere 2011, 83, 1320–1325. [Google Scholar] [CrossRef]
- Heo, J.-J.; Lee, J.-W.; Kim, S.-K.; Oh, J.-E. Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. J. Hazard. Mater. 2014, 279, 402–409. [Google Scholar] [CrossRef]
- Choo, G.; Oh, J.E. Seasonal occurrence and removal of organophosphate esters in conventional and advanced drinking water treatment plants. Water Res. 2020, 186, 116359. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ekpe, O.D.; Lee, H.J.; Oh, J.E. Perfluoroalkyl substances and pharmaceuticals removal in full-scale drinking water treatment plants. J. Hazard. Mater. 2020, 400, 123235. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.C.; Chang, R.W.; Williams, G.P.; Chang, Y.S.; Tomasko, D.; LaGory, K.; Ditmars, J.; Chun, H.D.; Lee, B.-K. Water quality issues in the Nakdong River Basin in the Republic of Korea. Environ. Eng. Policy 1999, 2, 131–143. [Google Scholar] [CrossRef]
- US EPA. National center for environmental assessment. In Exposure Factors Handbook; Moya, J., Ed.; EPA: Washington, DC, USA, 2011. [Google Scholar]
- NIER (National Institute of Environmental Research). Korean Exposure Factors Handbook; NIER: Incheon, Korea, 2019. [Google Scholar]
- NIER (National Institute of Environmental Research). Korean Exposure Factors Handbook for Children; NIER: Incheon, Korea, 2019. [Google Scholar]
- Seo, C.-D.; Son, H.-J.; Choi, J.-T.; Ryu, D.-C.; Yoo, P.-J. Occurrence of Organophosphorus Flame Retardants (OPFRs) in Nakdong River Basin: Mainstreams, Tributaries and STP Effluents. J. Korean Soc. Environ. Eng. 2015, 37, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Choo, G.; Cho, H.S.; Park, K.; Lee, J.W.; Kim, P.; Oh, J.E. Tissue-specific distribution and bioaccumulation potential of organophosphate flame retardants in crucian carp. Environ. Pollut. 2018, 239, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shen, X.; Liu, W.; Covaci, A.; Yang, F. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci. Total Environ. 2015, 538, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, N.; Zhang, B.; Jin, L.; Li, M.; Hu, M.; Zhang, X.; Wei, S.; Yu, H. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 2014, 54, 53–61. [Google Scholar] [CrossRef]
- MOE (Ministry of Environment). Checking and blocking sources of perfluoroalkyl substances detected in the Nakdong River (in Korean). 2018. Available online: https://www.me.go.kr/home/web/board/read.do?boardMasterId=1&boardId=876890&menuId=286 (accessed on 22 June 2018).
- Kim, H.Y.; Seok, H.W.; Kwon, H.O.; Choi, S.D.; Seok, K.S.; Oh, J.E. A national discharge load of perfluoroalkyl acids derived from industrial wastewater treatment plants in Korea. Sci. Total Environ. 2016, 563–564, 530–537. [Google Scholar] [CrossRef]
- Liang, K.; Liu, J. Understanding the distribution, degradation and fate of organophosphate esters in an advanced municipal sewage treatment plant based on mass flow and mass balance analysis. Sci. Total Environ. 2016, 544, 262–270. [Google Scholar] [CrossRef]
- Kim, U.-J.; Oh, J.K.; Kannan, K. Occurrence, Removal, and Environmental Emission of Organophosphate Flame Retardants/Plasticizers in a Wastewater Treatment Plant in New York State. Environ. Sci. Technol. 2017, 51, 7872–7880. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.G.; Liu, Y.S.; Ying, G.G. Perfluoroalkyl substances (PFASs) in wastewater treatment plants and drinking water treatment plants: Removal efficiency and exposure risk. Water Res. 2016, 106, 562–570. [Google Scholar] [CrossRef] [PubMed]




| Compound | n | Number of Cases According to Removal Efficiency Range | |||||
|---|---|---|---|---|---|---|---|
| ≥80% | <80% ≥50% | <50% ≥0% | <0% ≥−100% | <−100% ≥−200% | <−200% | ||
| TEP | 35 | 12 | 11 | 10 | 1 | 1 | 0 |
| TIBP | 32 | 1 | 10 | 12 | 6 | 0 | 3 |
| TNBP | 36 | 1 | 15 | 14 | 5 | 0 | 1 |
| TCEP | 36 | 0 | 17 | 15 | 2 | 1 | 1 |
| TCIPP | 36 | 1 | 19 | 14 | 2 | 0 | 0 |
| TDCIPP | 16 | 12 | 0 | 1 | 1 | 1 | 1 |
| TBOEP | 36 | 0 | 13 | 17 | 5 | 0 | 1 |
| TPhP | 33 | 9 | 8 | 3 | 1 | 0 | 12 |
| EHDPP | 36 | 18 | 11 | 6 | 0 | 0 | 1 |
| TEHP | 19 | 0 | 8 | 10 | 0 | 0 | 1 |
| ∑13OPFR | 36 | 0 | 18 | 16 | 1 | 1 | 0 |
| PFPeA | 28 | 4 | 0 | 1 | 18 | 0 | 5 |
| PFHxA | 36 | 0 | 0 | 7 | 29 | 0 | 0 |
| PFHpA | 36 | 2 | 0 | 11 | 23 | 0 | 0 |
| PFOA | 36 | 0 | 0 | 17 | 18 | 0 | 1 |
| PFNA | 36 | 11 | 0 | 10 | 14 | 1 | 0 |
| PFDA | 20 | 5 | 1 | 3 | 4 | 0 | 7 |
| PFUnDA | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| ∑10PFCAs | 36 | 0 | 0 | 15 | 21 | 0 | 0 |
| L-PFBS | 35 | 1 | 0 | 6 | 18 | 0 | 0 |
| L-PFPeS | 20 | 18 | 0 | 0 | 2 | 0 | 0 |
| L-PFHxS | 34 | 1 | 0 | 3 | 7 | 4 | 19 |
| B-PFHxS | 29 | 1 | 0 | 4 | 4 | 3 | 17 |
| L-PFOS | 34 | 11 | 0 | 10 | 11 | 1 | 1 |
| B-PFOS | 30 | 13 | 1 | 9 | 7 | 0 | 0 |
| L-PFNS | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| ∑9PFSAs | 36 | 0 | 2 | 6 | 12 | 7 | 9 |
| N-EtFOSAA | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| 6:2FTS | 9 | 2 | 0 | 2 | 0 | 0 | 5 |
| ∑5Precursors | 10 | 2 | 0 | 1 | 1 | 0 | 6 |
| ∑27PFAS | 36 | 0 | 0 | 12 | 18 | 6 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, W.; Choi, S.; Choo, G.; Yang, M.; Park, J.-H.; Oh, J.-E. Organophosphate Flame Retardants and Perfluoroalkyl Substances in Drinking Water Treatment Plants from Korea: Occurrence and Human Exposure. Int. J. Environ. Res. Public Health 2021, 18, 2645. https://doi.org/10.3390/ijerph18052645
Sim W, Choi S, Choo G, Yang M, Park J-H, Oh J-E. Organophosphate Flame Retardants and Perfluoroalkyl Substances in Drinking Water Treatment Plants from Korea: Occurrence and Human Exposure. International Journal of Environmental Research and Public Health. 2021; 18(5):2645. https://doi.org/10.3390/ijerph18052645
Chicago/Turabian StyleSim, Wonjin, Sol Choi, Gyojin Choo, Mihee Yang, Ju-Hyun Park, and Jeong-Eun Oh. 2021. "Organophosphate Flame Retardants and Perfluoroalkyl Substances in Drinking Water Treatment Plants from Korea: Occurrence and Human Exposure" International Journal of Environmental Research and Public Health 18, no. 5: 2645. https://doi.org/10.3390/ijerph18052645
APA StyleSim, W., Choi, S., Choo, G., Yang, M., Park, J. -H., & Oh, J. -E. (2021). Organophosphate Flame Retardants and Perfluoroalkyl Substances in Drinking Water Treatment Plants from Korea: Occurrence and Human Exposure. International Journal of Environmental Research and Public Health, 18(5), 2645. https://doi.org/10.3390/ijerph18052645