The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review
2.1.1. Search Strategy
2.1.2. Study Selection
2.1.3. Data Extraction
2.2. Pilot Study
2.2.1. The Mamma & Bambino Cohort
2.2.2. Assessment of Alcohol Consumption
2.2.3. Biological Samples
2.2.4. Measurement of Telomere Length
2.2.5. Estimation of Statistical Power
2.2.6. Statistical Analyses
3. Results
3.1. Systematic Review
3.1.1. Selection of Studies
3.1.2. Study Characteristics
3.1.3. Telomere Length in Patients with Alcohol-Related Disorders
3.1.4. Alcohol Consumption and Telomere Length
3.2. Pilot Study
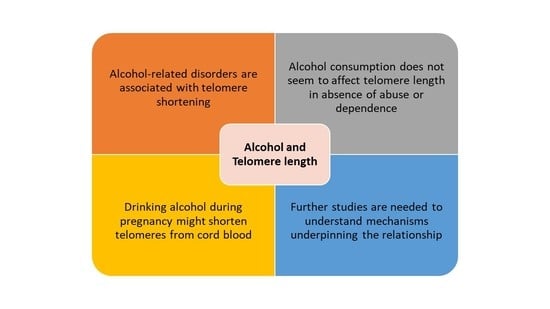
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, J.M.; Collins, K. Telomere maintenance and disease. Lancet 2003, 362, 983–988. [Google Scholar] [CrossRef]
- Mirabello, L.; Yu, K.; Kraft, P.; de Vivo, I.; Hunter, D.J.; Prescott, J.; Wong, J.Y.; Chatterjee, N.; Hayes, R.B.; Savage, S.A. The association of telomere length and genetic variation in telomere biology genes. Hum. Mutat. 2010, 31, 1050–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demissie, S.; Levy, D.; Benjamin, E.J.; Cupples, L.A.; Gardner, J.P.; Herbert, A.; Kimura, M.; Larson, M.G.; Meigs, J.B.; Keaney, J.F.; et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006, 5, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sun, D.; Ori, A.P.S.; Lu, A.T.; Seeboth, A.; Harris, S.E.; Deary, I.J.; Marioni, R.E.; Soerensen, M.; Mengel-From, J.; et al. Epigenome-wide association study of leukocyte telomere length. Aging 2019, 11, 5876–5894. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Mahdi, F. Telomere length variations in aging and age-related diseases. Curr. Aging Sci. 2014, 7, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.S.; van der Stukken, C.; Derom, C.; Thiery, E.; Bijnens, E.M.; Nawrot, T.S. Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child- and adulthood. EBioMedicine 2021, 63, 103164. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Shen, G.; Huang, J.Y.; Huang, Y.Q.; Feng, Y.Q. The Relationship between Telomere Length and Cancer Mortality: Data from the 1999–2002 National Healthy and Nutrition Examination Survey (NHANES). J. Nutr. Health Aging 2020, 24, 9–15. [Google Scholar] [CrossRef]
- Pan, W.; Du, J.; Shi, M.; Jin, G.; Yang, M. Short leukocyte telomere length, alone and in combination with smoking, contributes to increased risk of gastric cancer or esophageal squamous cell carcinoma. Carcinogenesis 2017, 38, 12–18. [Google Scholar] [CrossRef]
- Peng, H.; Zhu, Y.; Yeh, F.; Cole, S.A.; Best, L.G.; Lin, J.; Blackburn, E.; Devereux, R.B.; Roman, M.J.; Lee, E.T.; et al. Impact of biological aging on arterial aging in American Indians: Findings from the Strong Heart Family Study. Aging 2016, 8, 1583–1592. [Google Scholar] [CrossRef] [Green Version]
- Révész, D.; Verhoeven, J.E.; Milaneschi, Y.; Penninx, B.W. Depressive and anxiety disorders and short leukocyte telomere length: Mediating effects of metabolic stress and lifestyle factors. Psychol. Med. 2016, 46, 2337–2349. [Google Scholar] [CrossRef] [Green Version]
- Gorenjak, V.; Petrelis, A.M.; Stathopoulou, M.G.; Visvikis-Siest, S. Telomere length determinants in childhood. Clin. Chem. Lab. Med. 2020, 58, 162–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Song, L.; Zhang, L.; Wu, M.; Wang, L.; Cao, Z.; Xiong, C.; Zhang, B.; Li, Y.; Xia, W.; et al. Prenatal second-hand smoke exposure and newborn telomere length. Pediatr. Res. 2020, 87, 1081–1085. [Google Scholar] [CrossRef]
- Salihu, H.M.; King, L.M.; Nwoga, C.; Paothong, A.; Pradhan, A.; Marty, P.J.; Daas, R.; Whiteman, V.E. Association Between Maternal-Perceived Psychological Stress and Fetal Telomere Length. South. Med. J. 2016, 109, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Pradhan, A.; King, L.; Paothong, A.; Nwoga, C.; Marty, P.J.; Whiteman, V. Impact of intrauterine tobacco exposure on fetal telomere length. Am. J. Obstet. Gynecol. 2015, 212, 205.e1–205.e8. [Google Scholar] [CrossRef]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.B.P.; Gasparrini, A.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Plusquin, M.; Nawrot, T.S. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. 2017, 171, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Gutin, B.; Davis, C.L.; Keeton, D.; Thomas, J.; Stallmann-Jorgensen, I.; Mooken, G.; Bundy, V.; Snieder, H.; et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2011, 158, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, A.L.; Kronmal, R.A.; Kimura, M.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Hardikar, S.; Aviv, A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 421–429. [Google Scholar] [CrossRef] [Green Version]
- How science can put the Sustainable Development Goals back on track. Nature 2021, 589, 329–330. [CrossRef] [PubMed]
- Warren, K.R. A Review of the History of Attitudes Toward Drinking in Pregnancy. Alcohol Clin. Exp. Res. 2015, 39, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; la Rosa, M.C.; Agodi, A. Dietary Folate Intake and Folic Acid Supplements among Pregnant Women from Southern Italy: Evidence from the “Mamma & Bambino” Cohort. Int. J. Environ. Res. Public Health 2020, 17, 638. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, A.; Barchitta, M.; Agrifoglio, O.; Favara, G.; La Mastra, C.; la Rosa, M.C.; Magnano San Lio, R.; Panella, M.; Cianci, A.; Agodi, A. The impact of social determinants and lifestyles on dietary patterns during pregnancy: Evidence from the “Mamma & Bambino” study. Ann. Ig. 2019, 31. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Favara, G.; la Rosa, M.C.; La Mastra, C.; Magnano San Lio, R.; Agodi, A. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients 2019, 11, 1308. [Google Scholar] [CrossRef] [Green Version]
- Barchitta, M.; Maugeri, A.; la Rosa, M.C.; Magnano-San-Lio, R.; Favara, G.; Panella, M.; Cianci, A.; Agodi, A. Single Nucleotide Polymorphisms in Vitamin D Receptor Gene Affect Birth Weight and the Risk of Preterm Birth: Results From the “Mamma & Bambino” Cohort and A Meta-Analysis. Nutrients 2018, 10, 1172. [Google Scholar] [CrossRef] [Green Version]
- Magnano-San-Lio, R.; Maugeri, A.; la Rosa, M.C.; Cianci, A.; Panella, M.; Giunta, G.; Agodi, A.; Barchitta, M. The Impact of Socio-Demographic Factors on Breastfeeding: Findings from the “Mamma & Bambino” Cohort. Medicina 2021, 57, 103. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Giunta, G.; Cianci, A.; Agodi, A. Vaccination Status of Mothers and Children from the ‘Mamma & Bambino’ Cohort. Vaccines 2021, 9, 168. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; Favara, G.; la Rosa, M.C.; la Mastra, C.; Basile, G.; Agodi, A. Adherence to the Mediterranean diet partially mediates socioeconomic differences in leukocyte LINE-1 methylation: Evidence from a cross-sectional study in Italian women. Sci. Rep. 2020, 10, 14360. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Scalisi, A.; Agodi, A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients 2018, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, A.; Barchitta, M.; Fiore, V.; Rosta, G.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Agodi, A. Determinants of Adherence to the Mediterranean Diet: Findings from a Cross-Sectional Study in Women from Southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchitta, M.; Maugeri, A.; La Mastra, C.; Rosa, M.C.; Favara, G.; Lio, R.M.S.; Agodi, A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients 2020, 12, 1384. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Quattrocchi, A.; Agodi, A. Dietary Patterns are Associated with Leukocyte LINE-1 Methylation in Women: A Cross-Sectional Study in Southern Italy. Nutrients 2019, 11, 1843. [Google Scholar] [CrossRef] [Green Version]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes. Nutr. 2015, 10, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Barone, G.; Mazzoleni, P.; Catalfo, A.; De Guidi, G.; Iemmolo, M.G.; Crimi, N.; Agodi, A. Mediterranean Diet and Particulate Matter Exposure Are Associated With LINE-1 Methylation: Results from a Cross-Sectional Study in Women. Front. Genet. 2018, 9, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri, A.; Hlinomaz, O.; Agodi, A.; Barchitta, M.; Kunzova, S.; Bauerova, H.; Sochor, O.; Medina-Inojosa, J.R.; Lopez-Jimenez, F.; Vinciguerra, M.; et al. Is Drinking Alcohol Really Linked to Cardiovascular Health? Evidence from the Kardiovize 2030 Project. Nutrients 2020, 12, 2848. [Google Scholar] [CrossRef] [PubMed]
- Aida, J.; Yokoyama, A.; Izumiyama, N.; Nakamura, K.; Ishikawa, N.; Poon, S.S.; Fujiwara, M.; Sawabe, M.; Matsuura, M.; Arai, T.; et al. Alcoholics show reduced telomere length in the oesophagus. J. Pathol. 2011, 223, 410–416. [Google Scholar] [CrossRef]
- Aida, J.; Yokoyama, A.; Hara, S.; Ishizaki, T.; Fujiwara, M.; Arai, T.; Ishiwata, T.; Takubo, K. Telomere shortening in the oral epithelium in relation to alcohol intake, alcohol dehydrogenase (ADH-1B), and acetaldehyde dehydrogenase (ALDH-2) genotypes and clinicopathologic features. J. Oral Pathol. Med. 2020, 49, 82–90. [Google Scholar] [CrossRef]
- Dixit, S.; Whooley, M.A.; Vittinghoff, E.; Roberts, J.D.; Heckbert, S.R.; Fitzpatrick, A.L.; Lin, J.; Leung, C.; Mukamal, K.J.; Marcus, G.M. Alcohol consumption and leukocyte telomere length. Sci. Rep. 2019, 9, 1404. [Google Scholar] [CrossRef]
- Latifovic, L.; Peacock, S.D.; Massey, T.E.; King, W.D. The Influence of Alcohol Consumption, Cigarette Smoking, and Physical Activity on Leukocyte Telomere Length. Cancer Epidemiol. Biomark. Prev. 2016, 25, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; Prescott, J.; Giovannucci, E.; Hankinson, S.E.; Rosner, B.; de Vivo, I. One-carbon metabolism factors and leukocyte telomere length. Am. J. Clin. Nutr. 2013, 97, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Martins de Carvalho, L.; Wiers, C.E.; Manza, P.; Sun, H.; Schwandt, M.; Wang, G.J.; Grassi-Oliveira, R.; Godard, A.L.B.; Volkow, N.D. Effect of alcohol use disorder on cellular aging. Psychopharmacology 2019, 236, 3245–3255. [Google Scholar] [CrossRef]
- Needham, B.L.; Adler, N.; Gregorich, S.; Rehkopf, D.; Lin, J.; Blackburn, E.H.; Epel, E.S. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc. Sci. Med. 2013, 85, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavanello, S.; Hoxha, M.; Dioni, L.; Bertazzi, P.A.; Snenghi, R.; Nalesso, A.; Ferrara, S.D.; Montisci, M.; Baccarelli, A. Shortened telomeres in individuals with abuse in alcohol consumption. Int. J. Cancer 2011, 129, 983–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Révész, D.; Milaneschi, Y.; Terpstra, E.M.; Penninx, B.W. Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology 2016, 67, 153–162. [Google Scholar] [CrossRef]
- Shin, C.; Baik, I. Associations Between Alcohol Consumption and Leukocyte Telomere Length Modified by a Common Polymorphism of ALDH2. Alcohol Clin. Exp. Res. 2016, 40, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, T.E.; Strandberg, A.Y.; Saijonmaa, O.; Tilvis, R.S.; Pitkälä, K.H.; Fyhrquist, F. Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki Businessmen Study. Eur. J. Epidemiol. 2012, 27, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Mwangi, B.; Hasan, K.M.; Narayana, P.A.; Steinberg, J.L.; Walss-Bass, C.; Moeller, F.G.; Schmitz, J.M.; Lane, S.D. Measures of possible allostatic load in comorbid cocaine and alcohol use disorder: Brain white matter integrity, telomere length, and anti-saccade performance. PLoS ONE 2019, 14, e0199729. [Google Scholar] [CrossRef] [Green Version]
- Weischer, M.; Bojesen, S.E.; Nordestgaard, B.G. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 2014, 10, e1004191. [Google Scholar] [CrossRef]
- Yamaki, N.; Matsushita, S.; Hara, S.; Yokoyama, A.; Hishimoto, A.; Higuchi, S. Telomere shortening in alcohol dependence: Roles of alcohol and acetaldehyde. J. Psychiatr. Res. 2019, 109, 27–32. [Google Scholar] [CrossRef]
- Beach, S.R.; Dogan, M.V.; Lei, M.K.; Cutrona, C.E.; Gerrard, M.; Gibbons, F.X.; Simons, R.L.; Brody, G.H.; Philibert, R.A. Methylomic Aging as a Window onto the Influence of Lifestyle: Tobacco and Alcohol Use Alter the Rate of Biological Aging. J. Am. Geriatr. Soc. 2015, 63, 2519–2525. [Google Scholar] [CrossRef]
- Harpaz, T.; Abumock, H.; Beery, E.; Edel, Y.; Lahav, M.; Rozovski, U.; Uziel, O. The Effect of Ethanol on Telomere Dynamics and Regulation in Human Cells. Cells 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas-Simoes, T.M.; Ros, E.; Sala-Vila, A. Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism 2016, 65, 406–415. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Girchenko, P.; Lahti, J.; Czamara, D.; Knight, A.K.; Jones, M.J.; Suarez, A.; Hämäläinen, E.; Kajantie, E.; Laivuori, H.; Villa, P.M.; et al. Associations between maternal risk factors of adverse pregnancy and birth outcomes and the offspring epigenetic clock of gestational age at birth. Clin. Epigenet. 2017, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri, A.; Barchitta, M. A Systematic Review of Ecological Momentary Assessment of Diet: Implications and Perspectives for Nutritional Epidemiology. Nutrients 2019, 11, 2696. [Google Scholar] [CrossRef] [Green Version]


| Study | Study Design | Population | Age (Years) | Gender (% of Men) | Alcohol-Related Classification | Sample | Telomere Length Assessment |
|---|---|---|---|---|---|---|---|
| Aida et al., 2011 [37] | Cross-sectional | 26 alcoholic patients and 24 controls without head and neck, esophagus, stomach, or lung cancer | Mean of 61.2 in alcoholic patients and 73.3 in the control group | 100% of alcoholic patients and 50% in the control group | DSM-IV criteria for alcohol dependence | Esophageal mucosa | Quantitative fluorescence in situ hybridization |
| Aida et al., 2019 [38] | Cross-sectional | 21 subjects without head and neck, esophagus, stomach, or lung cancer | Mean of 40.4 | 57.1% | History of alcohol drinking classified as active drinking and non-active drinking. Active drinkers were also categorized as light drinkers and heavy drinkers | Oral epithelium | Quantitative fluorescence in situ hybridization |
| Dixit et al., 2019 [39] | Prospective | 1675 participants in the Heart and Soul Study and the Cardiovascular Health Study | Mean of 66.8 in the Heart and Soul Study and 74.8 in the Cardiovascular Health Study | 81.5% in the Heartand Soul Study and 41.2% in the Cardiovascular Health Study | Alcohol consumption; alcohol type; binge drinking; and ideal drinking | Blood | Southern blot analysis of terminal restriction fragment lengths |
| Latifovic et al., 2015 [40] | Cross-sectional | 477 healthy volunteers | 20–50 years | 43% | Alcohol consumption categorized into abstainer, low, moderate, and high | Blood | Quantitative real-time PCR |
| Liu et al., 2013 [41] | Cross-sectional | 1715 participants from the Nurses’ Health Study | Median of 59.8 | 0% | Alcohol intake obtained from Food Frequency Questionnaire | Blood | Quantitative real-time PCR |
| Martins de Carvalho et al., 2019 [42] | Cross-sectional | 260 patients with alcohol use disorder and 449 healthy controls | Mean of 44 in patients with alcohol use disorders and 33.3 in controls | 71.9% of patients with alcohol use disorder and 55.2% of controls | DSM-IV criteria for alcohol dependence and drinking behaviors | Blood | Quantitative real-time PCR |
| Needham et al., 2013 [43] | Cross-sectional | 5360 participants from the Nutrition Examination Survey | Mean of 48.6 | 48% | Alcohol use was classified as heavy and moderate drinking | Blood | Quantitative real-time PCR |
| Pavanello et al., 2011 [44] | Cross-sectional | 200 alcohol abusers and 257 controls | Mean of 38 in alcohol abusers and 44 in controls | 100% | Alcohol intake obtained from self-reported questionnaires | Blood | Quantitative real-time PCR |
| Révész et al., 2016 [45] | Prospective | 2936 participants from the Netherlands Study of Depression and Anxiety | 18–65 | 33.6% | Alcohol consumption obtained from questionnaires and categorized into non-drinking, mild–moderate drinking, and heavy drinking | Blood | Quantitative real-time PCR |
| Shin and Baik, 2016 [46] | Cross-sectional | 1771 participants from the Korean Genome Epidemiology Study | 49–79 | 49% | Alcohol consumption obtained from questionnaire-based interviews and categorized into light, moderate, and heavy consumption | Blood | Quantitative real-time PCR |
| Strandberg et al., 2012 [47] | Prospective | 499 men from the Helsinki Businessmen Study | Mean of 47.7 | 100% | Alcohol consumption obtained from questionnaire-based interviews | Blood | Southern blot analysis of terminal restriction fragment lengths |
| Tannous et al., 2019 [48] | Cross-sectional | 24 patients with alcohol use disorder and 25 controls | Mean of 47.0 in patients with alcohol use disorder and 43.8 in controls | 75% of patients with alcohol use disorder and 68% in controls | DSM-IV criteria for alcohol dependence | Blood | Quantitative real-time PCR |
| Weischer et al., 2014 [49] | Prospective | 4576 participants from the Copenhagen City Heart Study | 38–68 | 43% | Alcohol consumption obtained from self-reported questionnaire | Blood | Quantitative real-time PCR |
| Yamaki et al., 2018 [50] | Cross-sectional | 134 alcoholic patients (48 with upper aerodigestive tract cancer and 86 age-matched controls) and 121 non-alcoholic controls | 58.7% | 100% | Alcohol consumption obtained from the Kurihama Alcoholism Screening Test | Blood | Southern blot analysis of terminal restriction fragment lengths |
| Study | Main Results | Additional Findings |
|---|---|---|
| Aida et al., 2011 [37] | NTCR of basal cells was significantly larger in controls than in alcoholic patients | Basal cells had larger NTCR than parabasal cells |
| Aida et al., 2019 [38] | No difference in NTCR between non-drinkers and drinkers | No difference in NTCR between active or inactive ALDH2 genotypes |
| Dixit et al., 2019 [39] | At baseline and after 5 years of follow-up, TL was not different between alcohol consumers and alcohol abstainers. Weekly alcohol consumption did not correlate with TL | In Heart and Soul Study, binge drinking was associated with shorter TL. In Cardiovascular Health Study, no association between alcohol type and TL |
| Latifovic et al., 2015 [40] | No association between alcohol consumption and relative TL | Smoking status was associated with relative TL |
| Liu et al., 2013 [41] | No association between alcohol intake and relative TL | No relationships of folate, choline, methionine, riboflavin, vitamin B6, vitamin B12, and polymorphisms involved in one-carbon metabolism with relative TL |
| Martins de Carvalho et al., 2019 [42] | Alcohol use disorder was associated with lower relative TL. However, drinking behaviors were not associated with relative TL | A significant interaction between age and alcohol use disorder on relative telomere length was evident |
| Needham et al., 2013 [43] | No association between alcohol use and relative TL | The association between educational level and TL was partially mediated by smoking and body mass index but not by drinking or sedentary behavior |
| Pavanello et al., 2011 [44] | Relative TL was lower in alcohol abusers than in controls. The number of drinks per year was associated with relative TL in the overall population and among alcohol abusers | Polymorphisms in ADH1C and ALDH2 genes were not associated with TL |
| Révész et al., 2016 [45] | At the baseline, heavy drinking was associated with shorter TL if compared with moderate drinking | The association was not significant after adjusting for other predictors |
| Shin and Baik, 2016 [46] | No association between alcohol consumption and relative TL | An inverse association was found for heavy drinking among participants with mutant alleles of rs2074356 of ALDH2 gene |
| Strandberg et al., 2012 [47] | Age-adjusted TL was inversely associated with alcohol consumption at the baseline but not at the last follow-up | The association remained significant after adjusting for smoking, body mass index, cholesterol, perceived fitness |
| Tannous et al., 2019 [48] | Relative TL was lower in patients with alcohol disorder than in controls, but this difference was not statistically significant | NR |
| Weischer et al., 2014 [49] | No association between alcohol intake and TL | TL was associated with age, smoking status, body mass index, and physical inactivity |
| Yamaki et al., 2018 [50] | TL was shorter in patients with alcoholic disorders than controls | No association with cancer diagnosis, ADH1B and ALDH2 polymorphisms |
| Characteristics | Drinkers (n = 5) | Non-Drinkers (n = 10) | p-Value |
|---|---|---|---|
| Age (years) a | 38.1 (4.2) | 37.9 (3.9) | 0.934 |
| Gestational age at sampling (weeks) a | 16.1 (2.2) | 16.2 (2.3) | 0.937 |
| Prepregnancy BMI (kg/m2) a | 24.2 (3.8) | 24.0 (3.9) | 0.926 |
| Gestational age at delivery (weeks) a | 38.9 (2.1) | 39.1 (2.0) | 0.860 |
| Fetal sex (male/female) | 3/2 | 6/4 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Ferlito, M.; Giunta, G.; Panella, M.; Cianci, A.; et al. The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 5038. https://doi.org/10.3390/ijerph18095038
Maugeri A, Barchitta M, Magnano San Lio R, La Rosa MC, La Mastra C, Favara G, Ferlito M, Giunta G, Panella M, Cianci A, et al. The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy. International Journal of Environmental Research and Public Health. 2021; 18(9):5038. https://doi.org/10.3390/ijerph18095038
Chicago/Turabian StyleMaugeri, Andrea, Martina Barchitta, Roberta Magnano San Lio, Maria Clara La Rosa, Claudia La Mastra, Giuliana Favara, Marco Ferlito, Giuliana Giunta, Marco Panella, Antonio Cianci, and et al. 2021. "The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy" International Journal of Environmental Research and Public Health 18, no. 9: 5038. https://doi.org/10.3390/ijerph18095038
APA StyleMaugeri, A., Barchitta, M., Magnano San Lio, R., La Rosa, M. C., La Mastra, C., Favara, G., Ferlito, M., Giunta, G., Panella, M., Cianci, A., & Agodi, A. (2021). The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy. International Journal of Environmental Research and Public Health, 18(9), 5038. https://doi.org/10.3390/ijerph18095038