Use of Nonprescription and Prescription Drugs and Drug Information Sources among Breastfeeding Women in Japan: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Participants
2.2. Data Collection
2.3. Measurements
2.3.1. Breastfeeding
2.3.2. Use of Nonprescription and Prescription Drugs
2.3.3. Drug Information Sources
2.4. Data Analysis
3. Results and Discussion
3.1. Participants
3.2. Relationship between Medicine Use and Breastfeeding
3.3. Medicine Use for Nonprescription and Prescription Drugs
3.4. Sources of Drug Safety Information during Breastfeeding Period
3.5. Limitations of this Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Optimal Duration of Exclusive Breastfeeding. Available online: https://apps.who.int/iris/handle/10665/67219 (accessed on 8 July 2022).
- Gunn, J.; Lumley, J.; Chondros, P.; Young, D. Does an early postnatal check-up improve maternal health: Results from a randomised trial in Australian general practice. Br. J. Obstet. Gynaecol. 1998, 105, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Jayawickrama, H.S.; Amir, L.H.; Pirotta, M.V. GPs’ decision-making when prescribing medicines for breastfeeding women: Content analysis of a survey. BMC Res. Notes 2010, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Stultz, E.E.; Stokes, J.L.; Shaffer, M.L.; Paul, I.M.; Berlin, C.M. Extent of medication use in breastfeeding women. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2007, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Wilson, W.G. A 16-day-old breast-fed infant with metabolic acidosis caused by salicylate. Clin. Pediatrics 1981, 20, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Eldelman, A.I.S.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- de Ponti, M.; Stewart, K.; Amir, L.H.; Hussainy, S.Y. Medicine use and safety while breastfeeding: Investigating the perspectives of community pharmacists in Australia. Aust. J. Prim. Health 2015, 21, 46–57. [Google Scholar] [CrossRef]
- Jones, W.; Brown, D. The pharmacist’s contribution to primary care support for lactating mothers requiring medication. J. Soc. Adm. Pharm. 2000, 17, 88–98. [Google Scholar]
- Ronai, C.; Taylor, J.S.; Dugan, E.; Feller, E. The identifying and counseling of breastfeeding women by pharmacists. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2009, 4, 91–95. [Google Scholar] [CrossRef]
- MHLW. An Outline of the Japanese Medical System, Japanese Ministry of Health, Labour and Welfare (MHLW). Available online: https://www.mhlw.go.jp/english/policy/health-medical/health-insurance/index.html (accessed on 18 August 2022).
- MHLW. Annual Report of the Pharmaceutical Industry Production Dynamics Statistics, Japanese Ministry of Health, Labour and Welfare (MHLW). Available online: https://www.mhlw.go.jp/topics/yakuji/2020/nenpo/ (accessed on 18 August 2022).
- Arakawa, K.; Watanabe, S.; Hayashi, N.; Iwata, H.; Kobayashi, N.; Fujimoto, K.; Yamaura, K. Survey of Satisfaction and Needs for Provision of Information on Prescription Drugs. Iryo Yakugaku (Jpn. J. Pharm. Health Care Sci.) 2020, 46, 615–627. [Google Scholar] [CrossRef]
- Schrempp, S.; Ryan-Haddad, A.; Galt, K.A. Pharmacist counseling of pregnant or lactating women. J. Am. Pharm. Assoc. 1996, 41, 887–890. [Google Scholar] [CrossRef]
- Takagi, K.; Onda, M.; Iwaki, A.; Nishikawa, N.; Arakawa, Y. Analysis of Anxiety of Pregnant and Lactating Women Concerning Use of Drugs Using Text Mining. Iryo Yakugaku (Jpn. J. Pharm. Health Care Sci.) 2011, 37, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Moretti, M.E.; Collantes, A.; Chong, D.; Mazzotta, P.; Koren, G.; Merchant, S.S.; Ito, S. Choice of breastfeeding and physicians’ advice: A cohort study of women receiving propylthiouracil. Pediatrics 2000, 106, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Schirm, E.; Schwagermann, M.P.; Tobi, H.; de Jong-van den Berg, L.T. Drug use during breastfeeding. A survey from the Netherlands. Eur. J. Clin. Nutr. 2004, 58, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Hussainy, S.Y.; Dermele, N. Knowledge, attitudes and practices of health professionals and women towards medication use in breastfeeding: A review. Int. Breastfeed. J. 2011, 6, 11. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsuura, K.; Gao, P.; Hottenbacher, L.; Tokunaga, H.; Nishimura, K.; Imazu, Y.; Reissenweber, H.; Witt, C.M. Traditional Japanese Kampo Medicine: Clinical Research between Modernity and Traditional Medicine-The State of Research and Methodological Suggestions for the Future. Evid. Based Complementary Altern. Med. Ecam 2011, 2011, 513842. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Murashima, A. Pharmacotherapy Consultation - Pregnancy and Lactation, 2nd ed.; Nanzando: Tokyo, Japan, 2014. [Google Scholar]
- Koren, G.; Cairns, J.; Chitayat, D.; Gaedigk, A.; Leeder, S.J. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006, 368, 704. [Google Scholar] [CrossRef]
- MHLW. Publication of Revised ‘Precautions’ for Medicinal Products Containing Codeine Phosphate Hydrate, Dihydrocodeine Phosphate or Tramadol Hydrochloride, Ministry of Health, Labour and Welfare of Japan. 2019. Available online: https://www.mhlw.go.jp/hourei/doc/tsuchi/T190710I0060.pdf (accessed on 8 July 2022).
- EMA. Restrictions on Use of Codeine for Pain Relief in Children—CMDh Endorses PRAC Recommendation, European Medicines Agency. 2013. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/codeine-containing-medicines (accessed on 8 July 2022).
- FDA. FDA Drug Safety Communication: FDA Restricts Use of Prescription Codeine Pain and Cough Medicines and Tramadol Pain Medicines in Children; Recommends Against Use in Breastfeeding Women; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-restricts-use-prescription-codeine-pain-and-cough-medicines-and (accessed on 8 July 2022).
- Hurwitz, E.S.; Barrett, M.J.; Bregman, D.; Gunn, W.J.; Pinsky, P.; Schonberger, L.B.; Drage, J.S.; Kaslow, R.A.; Burlington, D.B.; Quinnan, G.V.; et al. Public Health Service study of Reye’s syndrome and medications. Report of the main study. Jama 1987, 257, 1905–1911. [Google Scholar] [CrossRef]
- Ito, S.; Blajchman, A.; Stephenson, M.; Eliopoulos, C.; Koren, G. Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am. J. Obstet. Gynecol. 1993, 168, 1393–1399. [Google Scholar] [CrossRef]
- Matheson, I.; Lunde, P.K.; Notarianni, L. Infant rash caused by paracetamol in breast milk? Pediatrics 1985, 76, 651–652. [Google Scholar] [CrossRef]
- Berlin, C.M., Jr.; Yaffe, S.J.; Ragni, M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatric Pharmacol. 1980, 1, 135–141. [Google Scholar]
- Bitzen, P.O.; Gustafsson, B.; Jostell, K.G.; Melander, A.; Wahlin-Boll, E. Excretion of paracetamol in human breast milk. Eur. J. Clin. Pharmacol. 1981, 20, 123–125. [Google Scholar] [CrossRef]
- Notarianni, L.J.; Oldham, H.G.; Bennett, P.N. Passage of paracetamol into breast milk and its subsequent metabolism by the neonate. Br. J. Clin. Pharmacol. 1987, 24, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaceci, S.; Giusti, A.; De Angelis, A.; Della Barba, M.I.; De Vincenti, A.Y.; Vellone, E.; Alvaro, R. Medications, “Natural” Products, and Pharmacovigilance during Breastfeeding: A Mixed-Methods Study on Women’s Opinions. J. Hum. Lact. 2016, 32, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, S.; Øvrebø, T.G.; Amble, N.M.S.; Poole, A.C.; Nordeng, H. Risk perception, beliefs about medicines and medical adherence among pregnant and breastfeeding women with migraine: Findings from a cross-sectional study in Norway. BMJ Open 2019, 9, e026690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Breastfed Only (n = 46) | Breastfed More Than Half (n = 51) | Formula-Fed More Than Half (n = 20) | Formula Fed-Only (n = 11) | p-Value Among/Between the Groups | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | Cochran–Armitage Test for Trend | Fisher’s Exact Test | |||
| (Total, N = 128) | ||||||||
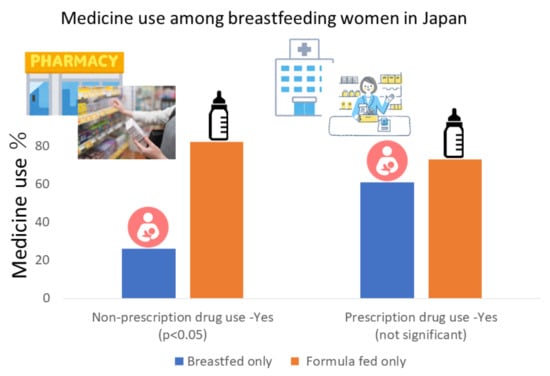
| Q1 | Nonprescription drug use among women whose children were in infancy | Yes | 12 (26) | 21 (41) | 11 (55) | 9 (82) | 0.0003 | 0.004 |
| Q2 | Prescription drug use among women whose children were in infancy | Yes | 28 (61) | 28 (55) | 16 (80) | 8 (73) | 0.1705 | 0.2303 |
| p-value between the questions (Fisher’s exact test) | 0.002 | 0.176 | 0.176 | 1.000 | ||||
| (Formula feeding group, n = 31) | ||||||||
| Q3 | Formula feeding because taking nonprescription drugs | Yes | n.a. | n.a. | 0 (0) | 0 (0) | n.a. | n.a. |
| Q4 | Formula feeding because taking prescription drugs | Yes | n.a. | n.a. | 0 (0) | 1 (9) | n.a. | 0.3548 |
| p-value between the questions (Fisher’s exact test) | n.a. | n.a. | n.a. | 1.000 | n.a. | |||
| (Breastfeeding group, n = 97) | ||||||||
| Q5 | Use of certain nonprescription drugs discontinued because of breastfeeding. | Yes | 34 (74) | 24 (47) | n.a. | n.a. | n.a. | 0.0079 |
| Q6 | Use of certain prescription drugs discontinued because of breastfeeding. | Yes | 20 (43) | 20 (39) | n.a. | n.a. | n.a. | 0.6853 |
| p-value between the questions (Fisher’s exact test) | 0.0056 | 0.5489 | n.a. | n.a. | n.a. | |||
| Type of Drugs a | Breastfeeding (BF) Group (n = 97) | Formula Feeding (FF) Group (n = 31) | Differenceb (BF–FF) | p-Value c | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | % | ||
| Cold medicines | 4 | 4 | 11 | 35 | −31 | <0.001 |
| Oral analgesics (medicines for headache and pain relief) | 7 | 7 | 8 | 26 | −19 | 0.009 |
| Drugs for menstrual pain | 0 | 0 | 4 | 13 | −13 | 0.003 |
| Digestive medicines | 1 | 1 | 4 | 13 | −12 | 0.012 |
| Drugs for skin blotches | 0 | 0 | 2 | 6 | −6 | 0.057 |
| Drugs for hay fever | 2 | 2 | 2 | 6 | −4 | 0.247 |
| Motion sickness drugs | 0 | 0 | 1 | 3 | −3 | 0.242 |
| Eczema drugs | 0 | 0 | 1 | 3 | −3 | 0.242 |
| Vitamin preparations | 4 | 4 | 2 | 6 | −2 | 0.632 |
| Anti-itching cream for insect bites/insect repellents | 1 | 1 | 1 | 3 | −2 | 0.427 |
| Vulnerary | 1 | 1 | 0 | 0 | 1 | 1.000 |
| Fomentation | 2 | 2 | 0 | 0 | 2 | 1.000 |
| Other supplements | 5 | 5 | 0 | 0 | 5 | 0.335 |
| Kampo medicine d | 15 | 15 | 3 | 10 | 6 | 0.559 |
| Type of Drugs a | Breastfeeding (BF) Group (n = 97) | Formula Feeding (FF) Group (n = 31) | Differenceb (BF–FF) | p-Value c | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | % | ||
| Cold medicines | 23 | 24 | 12 | 39 | −15 | 0.111 |
| Vitamins | 1 | 1 | 3 | 10 | −9 | 0.044 |
| Eczema drugs | 2 | 2 | 3 | 10 | −8 | 0.091 |
| Antiallergic drugs | 5 | 5 | 4 | 13 | −8 | 0.219 |
| Antiasthmatics | 2 | 2 | 2 | 6 | −4 | 0.247 |
| Thyroid drugs | 0 | 0 | 1 | 3 | −3 | 0.242 |
| Drugs for peptic ulcer/digestive medicines | 5 | 5 | 2 | 6 | −1 | 0.676 |
| Analgesic ointments/creams | 5 | 5 | 2 | 6 | −1 | 0.676 |
| Anti-infection drugs | 12 | 12 | 4 | 13 | −1 | 1.000 |
| Drugs for postpartum hemorrhage | 2 | 2 | 0 | 0 | 2 | 1.000 |
| Antihypertensives | 2 | 2 | 0 | 0 | 2 | 1.000 |
| Laxatives | 11 | 11 | 3 | 10 | 2 | 1.000 |
| Iron preparations | 8 | 8 | 1 | 3 | 5 | 0.687 |
| Oral analgesics | 31 | 32 | 8 | 26 | 6 | 0.655 |
| Kampo medicine d | 20 | 21 | 1 | 3 | 17 | 0.025 |
| Nonprescription Drugs | Prescription Drugs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Info. Sources | Drug Use | Info. Sources | Drug Use | |||||||||
| Category of Information Source (Multiple Choice Format a) | n | (%) d | Yes | (%) e | Odd Ratio b | p-Value c | n | (%) d | Yes | (%) e | Odd Ratio b | p-Value c |
| Prescribing doctor | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 60 | 62 | 52 | 87 | 54 | <0.001 |
| Other doctor (e.g., family doctor) | 10 | 10 | 7 | 70 | 5.5 | 0.03 | 4 | 4 | 1 | 25 | 0.2 | 0.30 |
| Dispensing pharmacist | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 18 | 19 | 16 | 89 | 7.8 | 0.003 |
| Pharmacist at the pharmacy medicine was purchased | 15 | 15 | 10 | 67 | 5.1 | 0.01 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Other pharmacists | 7 | 7 | 5 | 71 | 5.5 | 0.04 | 6 | 6 | 5 | 83 | 3.9 | 0.39 |
| Sales clerks f at the pharmacy of purchase | 2 | 2 | 2 | 100 | n.a. | 0.11 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Nurses/midwives | 4 | 4 | 3 | 75 | 6.3 | 0.11 | 6 | 6 | 5 | 83 | 3.9 | 0.39 |
| Administrative agency (e.g., health center) | 0 | 0 | 0 | n.a. | n.a. | n.a. | 0 | 0 | 0 | n.a. | n.a. | n.a. |
| Family/friends | 8 | 8 | 6 | 75 | 6.9 | 0.02 | 10 | 10 | 6 | 60 | 1.1 | 1.00 |
| Books | 1 | 1 | 0 | 0 | n.a. | 1.00 | 1 | 1 | 1 | 100 | n.a. | 1.00 |
| Internet | 21 | 22 | 8 | 38 | 1.3 | 0.80 | 28 | 29 | 19 | 68 | 1.8 | 0.25 |
| Drug package inserts | 6 | 6 | 4 | 67 | 4.2 | 0.17 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Not asked, not searched (including no opportunity to take medication) | 43 | 44 | 3 | 7 | n.a. | n.a. | 24 | 25 | 0 | 0 | n.a. | n.a. |
| Nonprescription Drugs | Prescription Drugs | |||||||
|---|---|---|---|---|---|---|---|---|
| Drug Use | Cochran–Armitage Test for Trend | Drug Use | Cochran–Armitage Test for Trend | |||||
| Total Number of Information Sources (Total of Multiple Choice Items) | n | Yes | (%) | p-Value | n | Yes | (%) | p-Value |
| <0.0001 | <0.0001 | |||||||
| 0 | 43 | 3 | 7 | 24 | 0 | 0 | ||
| 1 | 39 | 19 | 49 | 29 | 20 | 69 | ||
| 2 | 8 | 6 | 75 | 32 | 26 | 81 | ||
| 3 | 6 | 4 | 67 | 9 | 8 | 89 | ||
| 4 | 1 | 1 | 100 | 2 | 1 | 50 | ||
| 5 | 0 | n.a. | n.a. | 1 | 1 | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Hirokawa, K.; Kobuke, Y.; Kubota, T.; Yoshitake, T.; Haraguchi, K.; Honda, Y.; Kobayashi, H.; Harada, K.H. Use of Nonprescription and Prescription Drugs and Drug Information Sources among Breastfeeding Women in Japan: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 11722. https://doi.org/10.3390/ijerph191811722
Fujii Y, Hirokawa K, Kobuke Y, Kubota T, Yoshitake T, Haraguchi K, Honda Y, Kobayashi H, Harada KH. Use of Nonprescription and Prescription Drugs and Drug Information Sources among Breastfeeding Women in Japan: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(18):11722. https://doi.org/10.3390/ijerph191811722
Chicago/Turabian StyleFujii, Yukiko, Keiko Hirokawa, Yuko Kobuke, Toshio Kubota, Taketo Yoshitake, Koichi Haraguchi, Yukiko Honda, Hatasu Kobayashi, and Kouji H. Harada. 2022. "Use of Nonprescription and Prescription Drugs and Drug Information Sources among Breastfeeding Women in Japan: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 18: 11722. https://doi.org/10.3390/ijerph191811722
APA StyleFujii, Y., Hirokawa, K., Kobuke, Y., Kubota, T., Yoshitake, T., Haraguchi, K., Honda, Y., Kobayashi, H., & Harada, K. H. (2022). Use of Nonprescription and Prescription Drugs and Drug Information Sources among Breastfeeding Women in Japan: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(18), 11722. https://doi.org/10.3390/ijerph191811722