A Circular Economy Approach to Restoring Soil Substrate Ameliorated by Sewage Sludge with Amendments
Abstract
:1. Introduction
2. Materials and Methods
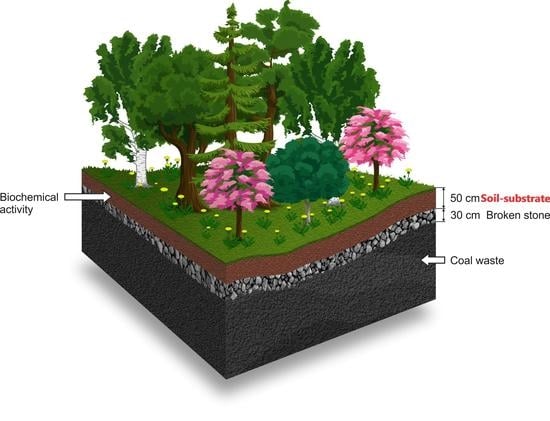
2.1. Study Area and Experimental Design
2.2. Sample Collection
2.3. An Investigation of Metal Concentrations in Soil
2.4. Concentrations of Metals in Plants
2.5. Water Content Percentage Measurement
2.6. Analysis of Plant Biochemical Parameters
2.7. Analyses of Enzymatic Activity
2.8. Soil-Substrate Physical Properties
2.9. Data Handling and Statistical Analysis
3. Results
- Pinus sylvestris—43.30%;
- Salix alba—34.57%;
- Acer negundo—46.71%;
- Robinia pseudoacacia—38.16%;
- Elaeagnus angustifolia—41.82%;
- Taraxacum officinale—67.09%;
- Tripleurospermum inodorum—70.20%.
4. Discussion
4.1. Assessment of the Phytomelioration Approach to Reclamation
4.2. Physiological Response of T. officinale to Abiotic Stress
4.3. Potentially Toxic Elements in Degraded Areas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wadgaonkar, S.L.; Nancharaiah, Y.V.; Esposito, G.; Lens, P.N.L. Environmental impact and bioremediation of seleniferous soils and sediments. Crit. Rev. Biotechnol. 2018, 38, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Halecki, W.; Klatka, S. Translocation of trace elements from sewage sludge amendments to plants in a reclaimed area. Bull. Environ. Contam. Toxicol. 2017, 99, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.M.; Santos, E.S.; Ferreira, M.; Magalhães, M.C.F. Cistus salviifolius a promising species for mine wastes remediation. J. Geochem. Explor. 2012, 113, 86–93. [Google Scholar] [CrossRef]
- Farahat, E.; Linderholm, W.H. The effect of long-term wastewater irrigation on accumulation and transfer of heavy metals in Cupressus sempervirens leaves and adjacent soils. Sci. Total Environ. 2015, 512–513, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Galal, T.M.; Shehata, H. Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanok, K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind. Crop. Prod. 2004, 19, 197–205. [Google Scholar] [CrossRef]
- Errington, I.; King, C.K.; Houlahan, S.; George, S.C.; Michie, A.; Hose, G.C. The influence of vegetation and soil properties on springtail communities in a diesel-contaminated soil. Sci. Total Environ. 2018, 619–620, 1098–1104. [Google Scholar] [CrossRef]
- Demková, L.; Jezný, T.; Bobuľská, L. Assessment of soil heavy metal pollution in a former mining area—Before and after the end of mining activities. Soil Water Res. 2017, 12, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Rad, S.; Xu, L.; Gui, L.; Song, X.; Li, Y.; Wu, Z.; Chen, Z. Heavy metals distribution, sources, and ecological risk assessment in Huixian Wetland, South China. Water 2020, 12, 431. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.C.; Nejad, Z.D.; Jung, M.C. Arsenic and heavy metals in paddy soil and polished rice contaminated by mining activities in Korea. Catena 2017, 148, 92–100. [Google Scholar] [CrossRef]
- Li, M.; Luo, Y.; Su, Z. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 2007, 147, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous impact of mining activities on soil heavy metals levels and human health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Landhausser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Quideau, S. Forest restoration following surface mining disturbance: Challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef] [Green Version]
- Asensio, V.; Forján, R.; Vega, F.A.; Covelo, E.F. Planting trees and amending with waste increases the capacity of mine tailings soils to retain Ni, Pb and Zn. Span. J. Soil. Sci. 2014, 4, 225–238. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E.; Gambuś, F.; Muszyńska, E.; Czech, T. Phytostabilization of Zn-Pb ore flotation tailings with Dianthus carthusianorum and Biscutella laevigata after amending with mineral fertilizers or sewage sludge. J. Environ. Manag. 2017, 189, 75–83. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; pp. 111–139. [Google Scholar]
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Ahamd, W.; Numan, M.; Shah, M.; Ahmad, I. Phytostabalization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J. Clean. Prod. 2020, 255, 120318. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef] [Green Version]
- De la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Ogagaoghene, A.J. pH level, ascorbic acid, proline and soluble sugar as bio-indicators for pollution. ChemSearch J. 2017, 8, 41–49. [Google Scholar]
- Hao, W.; Arora, R.; Yadav, A.K.; Joshee, N. Freezing tolerance and cold acclimation in guava (Psidium guajva L.). Hortscience 2009, 44, 1258–1266. [Google Scholar] [CrossRef]
- Kabata–Pendias, A. Trace Elements in Soils and Plants; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Pavlovic, P.; Tsigaridas, K. Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollut. 2011, 159, 3560–3570. [Google Scholar] [CrossRef]
- Halecki, W.; Klatka, S. Long term growth of crop plants on experimental plots created among slag heaps. Ecotoxicol. Environ. Saf. 2018, 147, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blainski, A.; Lopes, G.C.; Palazzo de Mello, J.C. Application and analysis of the FolinCiocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Lukatkin, A.S.; Anjum, N.A. Control of cucumber (Cucumis sativus L.) tolerance to chilling stress-evaluating the role of ascorbic acid and glutathione. Front. Environ. Sci. 2014, 2, 62. [Google Scholar] [CrossRef] [Green Version]
- Maleva, M.; Garmash, E.; Chukina, N.; Malec, P.; Waloszek, A.; Strzałka, K. Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brazilian elodea (Egeria densa (Planch.) Casp.) exposed to cadmium and manganese. Ecotox. Environ. Safe 2018, 160, 197–206. [Google Scholar] [CrossRef]
- Kaya, G.; Yaman, M. Determination of trace metals in plant leaves as biomonitor of pollution extent by a sensitive STAT–ASS method. Instrum. Sci. Technol. 2012, 40, 61–74. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 4, 555–559. [Google Scholar] [CrossRef]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil. 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Wösten, J.H.M.; van Genuchten, M.T. Division s-6-soil and water management and conservation. Using texture and other soil properties to predict the unsaturated soil hydraulic functions. Soil Sci. Soc. Am. J. 1988, 52, 1762–1770. [Google Scholar] [CrossRef]
- Dedousis, A.P.; Bartzanas, T. (Eds.) Soil Engineering (Soil Biology 20); Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Hernandez, T.; Moreno Juan, I.; Costa, F. Influence of sewage sludge application on crop yelds and heavy metal availability. J. Soil Sci. Plant Nutr. 1991, 37, 201–210. [Google Scholar] [CrossRef]
- Halecki, W.; Gąsiorek, M.; Gambuś, F.; Abram, R. The potential of hydrated and dehydrated sewage sludge discharges from soil reclamation appliances. Fresenius Environ. Bull. 2016, 25, 1935–1941. [Google Scholar]
- Joniec, J.; Gąsior, J.; Voloshanska, S.; Nazarkiewicz, M.; Hoivanovych, N. Evaluation of the effectiveness of land reclamation based on microbiological and biochemical parameters assessed in an ozokerite mining and processing landfill sown with Trifolium hybridum and Dactylis glomerata. J. Environ. Manag. 2019, 242, 343–350. [Google Scholar] [CrossRef]
- Lorestani, B.; Cheraghi, M.; Yousefi, N. Phytoremediation potential of native plants growing on a heavy metal contaminated soil of copper mine in Iran. World Acad. Sci. Eng. Technol. 2011, 77, 377–382. [Google Scholar]
- Isahak, A.; Surif, S.; Sahani, M.; Gill, A.; Phang, J. Environmental stewardship for gold mining in tropical regions. J. Degrad. Min. Lands Manag. 2013, 1, 37–42. [Google Scholar]
- Humsa, T.Z.; Srivastava, R.K. Impact of rare earth mining and processing on soil and water environment at Chavara, Kollam, Kerala: A case study. Procedia Earth Planet Sci. 2015, 11, 566–581. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Liao, X.; Yan, X.; Zhu, G.; Ma, D. Evaluation of heavy metal and polycyclic aromatic hydrocarbons accumulation in plants from typical industrial sites: Potential candidate in phytoremediation for co–contamination. Environ Sci. Pollut. Res. 2014, 21, 12494–12504. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Hanus–Fajerska, E.; Muszyńska, E.; Ciarkowska, K. Natural organic amendments for improved phytoremediation of polluted soils. A review of recent progress. Pedosphere 2015, 26, 1–12. [Google Scholar] [CrossRef]
- Tripath, P.; Chandra, A.; Prakash, J. Physio-biochemical assessment and expression analysis of genes associated with drought tolerance in sugarcane (Saccharum spp. hybrids) exposed to GA 3 at grand growth stage. Plant Biol. 2019, 21, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Altinkut, A.; Kazan, K.; Ipekci, Z.; Gozukirmizi, N. Tolerance to paraquat is correlated with the traits associated with water stress tolerance in segregating F2 populations of barley and wheat. Euphytica 2001, 121, 81–86. [Google Scholar] [CrossRef]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef]
- Li, H.; Wei, Z.; Wang, X.; Xu, F. Spectral characteristics of reclaimed vegetation in a rare earth mine and analysis of its correlation with the chlorophyll content. J. Appl. Spectrosc. 2020, 87, 553–562. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Fu, B.; Qi, Y.B.; Chang, Q.R. Impacts of revegetation management modes on soil properties and vegetation ecological restoration in degraded sandy grassland in farming-pastoral ecotone. Int. J. Agric. Biol. Eng. 2015, 8, 26–34. [Google Scholar]
- Kanzler, M.; Bohm, C.; Freese, D. Impact of P fertilization on the growth performance of black locust (Robinia pseudoacacia L.) in a lignite post–mining area in Germany. Ann. For. Res. 2015, 58, 39–54. [Google Scholar] [CrossRef]
- Odigie, K.O.; Rojero, J.; Hibdon, S.A.; Flegal, A.R. Natural lead levels in dandelions (Taraxacum officinale): A weed, folk medicine, and biomonitor. Environ. Sci. Technol. 2018, 53, 954–962. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Winkler, J.; Adamcová, D.; Radziemska, M.; Uldrijan, D.; Zloch, J. Municipal solid waste landfill–vegetation succession in an area transformed by human impact. Ecol. Eng. 2019, 129, 109–114. [Google Scholar] [CrossRef]
- Królak, E.; Marciniuk, J.; Popijantus, K.; Wasilczuk, P.; Kasprzykowski, Z. Environmental Factors Determining the Accumulation of Metals: Cu, Zn, Mn and Fe in Tissues of Taraxacum sp. sect. Taraxacum. Bull. Environ. Contam. Toxicol. 2018, 101, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Vanni, G.; Cardelli, R.; Marchini, F.; Saviozzi, A.; Guidi, L. Are the physiological and biochemical characteristics in dandelion plants growing in an urban area (Pisa, Italy) indicative of soil pollution? Water Air Soil Pollut. 2015, 226, 124–139. [Google Scholar] [CrossRef]
- Bretzel, F.; Benvenuti, S.; Pistelli, L. Metal contamination in urban street sediment in Pisa (Italy) can affect the production of antioxidant metabolites in Taraxacum officinale Weber. Environ. Sci. Pollut. Res. 2014, 21, 2325–2333. [Google Scholar] [CrossRef]
- Savinov, A.B.; Kurganova, L.N.; Shekunov, Y.I. Lipid peroxidation rates in Taraxacum officinale Wigg. and Vicia cracca L. from biotopes with different levels of soil pollution with heavy metals. Russ. J. Ecol. 2007, 38, 174–180. [Google Scholar] [CrossRef]
- Ligocki, M.; Tarasewicz, Z.; Anisko, M. The common dandelion (Taraxacum officinale) as an indicator of anthropogenic toxic metal pollution of environment. Acta Sci. Polonorum. Zootechnica. 2011, 10, 73–81. [Google Scholar]
- Malizia, D.; Giuliano, A.; Ortaggi, G.; Masotti, A. Common plants as alternative analytical tools to monitor heavy metals in soil. Chem. Cent. J. 2012, 6, S6. [Google Scholar] [CrossRef] [Green Version]
- Joniec, J.; Oleszczuk, P.; Jezierska-Tysa, S.; Kwiatkowska, E. Effect of reclamation treatments on microbial activity and phytotoxicity of soil degraded by the sulphur mining industry. Environ. Pollut. 2019, 252, 1429–1438. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Smolinska, B.; Leszczynska, J. Photosynthetic pigments and peroxidase activity of Lepidium sativum L. during assisted Hg phytoextraction. Environ. Sci. Pollut. Res. 2017, 24, 13384–13393. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.A.; Han, T.; Ahn, S.K.; Kang, H.; Cho, M.R.; Lee, S.C.; Im, K.H. Effects of heavy metals on plant growths and pigment contents in Arabidopsis thaliana. Plant Pathol. J. 2012, 28, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, L.; Siddiqui, Z.A.; Abd_Allah, E.F. Effects of interaction of Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani on the growth, chlorophyll, carotenoid and proline contents of carrot in three types of soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 324–331. [Google Scholar] [CrossRef]
- Alam, M.Z.; Carperter-Boggs, L.; Hoque, M.A.; Ahammed, G.J. Effect of soil amendments on antioxidant activity and photosyntheticpigments in pea crops grown in arsenic contaminated soil. Helyon 2020, 6, e05475. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Szepesi, A.; Szőllősi, R. Mechanism of proline biosynthesis and role of proline metabolism enzymes under environmental stress in plants. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Riyadh, Saudia Arabia, 2018; pp. 337–353. [Google Scholar]
- Petrovic, D.; Krivokapic, S. The effect of Cu, Zn, Cd, and Pb accumulation on biochemical parameters (proline, chlorophyll) in the water caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plants 2020, 9, 1287. [Google Scholar] [CrossRef]
- Fiasconaro, M.L.; Lovato, M.E.; Antolín, M.C.; Clementi, L.A.; Torres, N.; Gervasio, S.; Martín, C.A. Role of proline accumulation on fruit quality of pepper (Capsicum annuum L.) grown with a K-rich compost under drought conditions. Sci. Hortic. 2019, 249, 280–288. [Google Scholar] [CrossRef]
- Buhăianu, S.; Jităreanu, D.C. The relationship between chlorophyll content and antioxidant activity of Abies alba and Nepeta pannonica extracts according to phenophase and harvesting area. Cercet. Agron. Mold. 2019, 52, 158–165. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Sucharova, J.; Suchara, I.; Hola, M.; Reimann, C.; Boyd, R.; Filzmoser, P.; Englmaier, P. Linking chemical elements in forest floor humus (Oh-horizon) in the Czech Republic to contamination sources. Environ. Pollut. 2011, 159, 1205–1214. [Google Scholar] [CrossRef]
- Kaddour, K.; Smail, M.; Hocine, B.; Bouzaza, A.; El Hacen, B. Assessment of Heavy Metal Pollution Due to the Lead-Zinc Mine at the Ain Azel Area (northeast of Algeria). J. Environ. Manag. 2017, 8, 1. [Google Scholar] [CrossRef]
- Kleckerová, A.; Dočekalová, H. Dandelion plants as a biomonitor of urban area contamination by heavy metals. Int. J. Environ. Res. 2014, 8, 157–164. [Google Scholar]
- Zarzycki, J.; Petryk, A. The influence of soil properties and plant species’ composition on metal uptake by vegetation on fallow land. Fresenius Environ. Bull. 2018, 27, 2867–2872. [Google Scholar]
- Aničić, M.; Spasić, T.; Tomašević, M.; Rajšić, S.; Tasić, M. Trace elements accumulation and temporal trends in leaves of urban deciduous trees (Aesculus hippocastanum and Tilia spp.). Ecol. Indic. 2011, 11, 824–830. [Google Scholar] [CrossRef]
- Dogan, Y.; Unver, C.M.; Ugulu, I.; Calis, M.; Durkan, N. Heavy metal accumulation in the bark and leaves of Juglans regia planted in Artvin City. Turk. Biotech. Biotechnol. Equip. 2014, 28, 643–649. [Google Scholar] [CrossRef]
- Adamcová, D.; Vaverková, M.D.; Břoušková, E. The toxicity of two types of sewage sludge from wastewater treatment plant for plants. J. Ecol. Eng. 2016, 17, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Giacomino, A.; Malandrino, M.; Colombo, M.L.; Miaglia, S.; Maimone, P.; Blancato, S.; Abollino, O. Metal content in dandelion (Taraxacum officinale) leaves: Influence of vehicular traffic and safety upon consumption as food. J. Chem. 2016, 2016, 9842987. [Google Scholar] [CrossRef] [Green Version]
- Kalembasa, D.; Malinowska, E. The yield and content of trace elements in biomass of Miscanthus Sacchariflorus (Maxim.) Hack. and in soil in the third year of a pot experiment. J. Elementol. 2009, 14, 685–691. [Google Scholar] [CrossRef]
- Majtkowski, W.; Majtkowska, G. Fitosanitarna rola szaty roślinnej na zrekultywowanej hałdzie popiołów w Sowlanach k. Białegostoku. Biul. Inst. Hod. Aklim. Roślin 2012, 263, 55–63. (In Polish) [Google Scholar]
- Grekhova, I.; Gilmanova, M. The usage of sludge of wastewater in the composition of the soil for land reclamation. Procedia Eng. 2016, 165, 794–799. [Google Scholar] [CrossRef]





| Layer | Mass Water Content | Bulk Density | Volumetric Water Content | Total Porosity | Capillary Porosity | Soil Moisture | Organic Mater Content | Soil Texture |
|---|---|---|---|---|---|---|---|---|
| cm | g·g−1 | g·cm−3 | cm−3·cm−3 | % | % | % | % | |
| 10 | 0.41 | 1.54 | 0.45 | 44.11 | 38.40 | 36.35 | 28.99 | sandy clay loam |
| 20 | 0.37 | 1.47 | 0.48 | 48.17 | 37.61 | 30.25 | 29.53 | sandy clay loam |
| 30 | 0.45 | 1.55 | 0.49 | 49.30 | 39.84 | 23.32 | 25.50 | sandy clay loam |
| 50 | 0.43 | 1.57 | 0.52 | 49.18 | 40.00 | 20.41 | 23.00 | sandy clay loam |
| PTEs | Cr | Ni | Cu | Zn | Cd | Pb | Mn | Fe |
|---|---|---|---|---|---|---|---|---|
| Soil Substrate | ||||||||
| Pinus sylvestris | 45.24 | 76.93 | 36.65 | 177.91 | 5.86 | 84.10 | 78.53 | 13.34 |
| Salix alba | 38.76 | 34.86 | 45.53 | 431.02 | 6.10 | 98.54 | 41.23 | 14.40 |
| Betula pendula | 28.45 | 51.32 | 29.02 | 384.21 | 5.03 | 74.84 | 35.12 | 27.21 |
| Acer negundo | 78.89 | 60.38 | 91.25 | 466.92 | 4.25 | 141.13 | 34.67 | 26.08 |
| Robinia pseudoacacia | 41.63 | 45.89 | 78.67 | 343.09 | 7.90 | 97.05 | 35.45 | 17.65 |
| E. angustifolia | 53.55 | 54.54 | 64.55 | 355.54 | 8.69 | 36.45 | 61.95 | 16.43 |
| Taraxacum officinale | 53.63 | 43.25 | 23.95 | 278.30 | 2.94 | 78.76 | 0.87 | 20.76 |
| Tripleurospermum inodorum | 45.54 | 35.75 | 26.97 | 357.76 | 3.98 | 98.08 | 0.90 | 18.98 |
| Plants | ||||||||
| Pinus sylvestris | 4.54 | 17.45 | 3.46 | 100.49 | 0.89 | 13.90 | 46.78 | 0.14 |
| Salix alba | 22.65 | 45.67 | 2.54 | 366.6 | 0.48 | 5.62 | 78.34 | 0.65 |
| Betula pendula | 12.45 | 19.41 | 6.32 | 98.14 | 0.61 | 1.43 | 73.14 | 0.24 |
| Acer negundo | 45.54 | 1.63 | 9.43 | 32.43 | 0.54 | 5.74 | 26.45 | 0.74 |
| Robinia pseudoacacia | 2.54 | 3.65 | 7.46 | 26.65 | 0.75 | 0.57 | 12.53 | 0.45 |
| E. angustifolia | 4.12 | 1.67 | 12.45 | 28.56 | 0.98 | 4.94 | 41.05 | 0.46 |
| Taraxacum officinale | 12.43 | 34.54 | 23.94 | 84.93 | 0.57 | 34.65 | 0.46 | 35.54 |
| Tripleurospermum inodorum | 13.87 | 24.76 | 16.76 | 89.97 | 3.86 | 35.87 | 0.57 | 27.76 |
| Species | Invertase (mg Invertasied Sugar kg Soil/24 h) | Urease (mg N-NH4/kg/2 h) | Dehydrogenase (mg TPF/kg Soil/24 h) |
|---|---|---|---|
| Pinus sylvestris | 0.14 | 77.98 | 289.69 |
| Betula pendula | 0.09 | 90.84 | 54.7 |
| R. pseudoacacia | 0.01 | 36.75 | 12.76 |
| E. angustifolia | 0.36 | 40.35 | 36.97 |
| Taraxacum officinale | 0.21 | 35.21 | 13.43 |
| Tripleurospermum inodorum | 1.35 | 86.39 | 17.76 |
| Soil substrate | 0.23 | 52.92 | 13.01 |
| Plant Species | Chlorophyll a | Chlorophyll b | Carotenoids | Proline | TF | TPC |
|---|---|---|---|---|---|---|
| (mg/g) DW | (mg/g) DW | (mg/g) DW | (µmol/g) DW | (mg/eqC/g) DW | (mg/eq GA/g) DW | |
| T. officinale | 15.08–43.88 (31.79) * | 3.25–16.14 (10.17) | 53.09–461.49 (215.56) | 15.76–20.15 (17.64) | 1.93–2.52 (2.32) | 3.29–4.68 (3.89) |
| T. officinale (control site) | 10.97–29.72 (23.51) | 3.17–10.16 (5.12) | 55.43–914.37 (354.74) | 7.58–17.22 (13.14) | 0.27 –2.95 (1.32) | 3.03–5.54 (3.82) |
| Variable | Slope | Error | Intercept | Error | r | p |
|---|---|---|---|---|---|---|
| Carotenoids | −3.69 | 2.77 | 204.05 | 62.43 | −0.23 | 0.19 |
| Chlorophyll a | 0.03 | 0.02 | 1.88 | 0.45 | 0.24 | 0.17 |
| Chlorophyll b | −0.13 | 0.06 | 6.86 | 1.43 | −0.34 | 0.05 |
| TPC | −0.39 | 0.18 | 21.83 | 4.16 | −0.35 | 0.04 |
| TF | −0.02 | 0.02 | 2.43 | 0.35 | −0.27 | 0.12 |
| Mn | 1.06 | 0.25 | −7.66 | 5.58 | 0.62 | 0.03 |
| Cr | −1.69 | 2.67 | 110.73 | 6.43 | −0.11 | 0.53 |
| Fe | −0.43 | 0.12 | 1.65 | 2.73 | −0.54 | 0.32 |
| Ni | −0.71 | 0.33 | 3.63 | 7.36 | −0.36 | 0.04 |
| Cu | −1.27 | 0.36 | 58.42 | 8.04 | −0.53 | 0.32 |
| Zn | −3.91 | 1.67 | 238.83 | 37.69 | −0.38 | 0.03 |
| Cd | −0.11 | 0.04 | 4.97 | 0.85 | −0.46 | 0.01 |
| Pb | −2.06 | 0.55 | 81.52 | 12.34 | −0.55 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halecki, W.; López-Hernández, N.A.; Koźmińska, A.; Ciarkowska, K.; Klatka, S. A Circular Economy Approach to Restoring Soil Substrate Ameliorated by Sewage Sludge with Amendments. Int. J. Environ. Res. Public Health 2022, 19, 5296. https://doi.org/10.3390/ijerph19095296
Halecki W, López-Hernández NA, Koźmińska A, Ciarkowska K, Klatka S. A Circular Economy Approach to Restoring Soil Substrate Ameliorated by Sewage Sludge with Amendments. International Journal of Environmental Research and Public Health. 2022; 19(9):5296. https://doi.org/10.3390/ijerph19095296
Chicago/Turabian StyleHalecki, Wiktor, Nuria Aide López-Hernández, Aleksandra Koźmińska, Krystyna Ciarkowska, and Sławomir Klatka. 2022. "A Circular Economy Approach to Restoring Soil Substrate Ameliorated by Sewage Sludge with Amendments" International Journal of Environmental Research and Public Health 19, no. 9: 5296. https://doi.org/10.3390/ijerph19095296
APA StyleHalecki, W., López-Hernández, N. A., Koźmińska, A., Ciarkowska, K., & Klatka, S. (2022). A Circular Economy Approach to Restoring Soil Substrate Ameliorated by Sewage Sludge with Amendments. International Journal of Environmental Research and Public Health, 19(9), 5296. https://doi.org/10.3390/ijerph19095296