HR+/HER2– Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review
Abstract
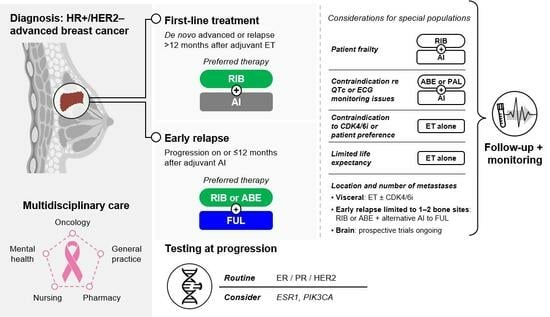
:1. Introduction
2. Methods
3. Results
3.1. Preferred Therapy for HR+/HER2– Advanced Breast Cancer
3.1.1. What Is the Preferred Treatment Combination in the First-Line HR+/HER2– Metastatic Setting?
3.1.2. What If the Patient Relapsed on or within 12 Months after Completion of Adjuvant AI?
3.1.3. In Which Situations Should an Alternative to Preferred Therapy Be Used?
3.1.4. What Phenotyping or Mutational Testing Should Be Undertaken at the Time of Progression?
3.1.5. What Are Best Practices for Monitoring Patients on CDK4/6i?
- Complete Blood Count
- Electrocardiogram
- Liver Function Tests
- Diarrhea
- Other
3.2. Special Populations
3.2.1. What about Preferred Treatments for ‘Fit’ Elderly Patients?
3.2.2. What about Preferred Treatments for Patients with Visceral Disease?
3.2.3. What about Practical Considerations for Other Special Populations, like Those with Brain Metastases or Those with Oligometastatic Disease?
3.3. Patient Considerations
What Is Important to Patients When Considering Treatment Options?
3.4. The Breast Cancer Team
Which Allied Health Care Partners Can Play a Role in the Care of Patients with Advanced Breast Cancer?
- Pharmacy
- Nursing
- General practice
- Mental health
4. Practical Application and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- CCS. Breast Cancer Statistics. Available online: https://cancer.ca/en/cancer-information/cancer-types/breast/statistics (accessed on 1 May 2022).
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 Mg versus Anastrozole 1 Mg for Hormone Receptor-Positive Advanced Breast Cancer (FALCON): An International, Randomised, Double-Blind, Phase 3 Trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Linden, H.M.; et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N. Eng. J. Med. 2019, 380, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus Exemestane for Hormone-Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Advanced Breast Cancer: Overall Survival Results from BOLERO-2. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef]
- Health Canada Approves New First-Line Treatment for Women Living with Metastatic Breast Cancer. Available online: https://www.trioncology.org/news/health-canada-approves-new-first-line-treatment-women-living-metastatic-breast-cancer/ (accessed on 10 May 2022).
- Health Canada Approves KISQALITM for the Treatment of HR-Positive and HER2-Negative Metastatic Breast Cancer in Postmenopausal Women in Combination with Letrozole as an Initial Endocrine-Based Therapy. Available online: https://www.newswire.ca/news-releases/health-canada-approves-kisqali-for-the-treatment-of-hr-positive-and-her2-negative-metastatic-breast-cancer-in-postmenopausal-women-in-combination-with-letrozole-as-an-initial-endocrine-based-therapy-681481661.html (accessed on 2 May 2018).
- Health Canada Approves New Drug to Treat Metastatic Breast Cancer through International and Domestic Joint Reviews. Available online: https://www.canada.ca/en/health-canada/news/2019/04/health-canada-approves-new-drug-to-treat-metastatic-breast-cancer-through-international-and-domestic-joint-reviews.html (accessed on 9 April 2019).
- Health Canada Approves an Extended Indication for KISQALI® (ribociclib) in Combination Treatment. Available online: https://www.newswire.ca/news-releases/health-canada-approves-an-extended-indication-for-kisqali-r-ribociclib-in-combination-treatment-812384479.html (accessed on 16 April 2020).
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus Letrozole as First-Line Therapy in Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer with Extended Follow-Up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Eng. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Eng. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Chavez Mac Gregor, M.; Bananis, E.; et al. Overall Survival (OS) with First-Line Palbociclib plus Letrozole (PAL+LET) versus Placebo plus Letrozole (PBO+LET) in Women with Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer (ER+/HER2−ABC): Analyses. J. Clin. Oncol. 2022, 40 (Suppl. S17), LBA1003. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.H.; Campone, M.; Chen, S.C.; Sanchez, L.M.M.; Paluch-Shimon, S.; et al. LBA15 MONARCH 3: Interim Overall Survival (OS) Results of Abemaciclib plus a Nonsteroidal Aromatase Inhibitor (NSAI) in Patients (Pts) with HR+, HER2- Advanced Breast Cancer (ABC). Ann. Oncol. 2022, 33, S1384. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus Endocrine Therapy for Premenopausal Women with Hormone-Receptor-Positive, Advanced Breast Cancer (MONALEESA-7): A Randomised Phase 3 Trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Eng. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2. JAMA Oncol. 2020, 6, 116. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus Palbociclib versus Fulvestrant plus Placebo for Treatment of Hormone-Receptor-Positive, HER2-Negative Metastatic Breast Cancer That Progressed on Previous Endocrine Therapy (PALOMA-3): Final Analysis of the Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; Demichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Eng. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Chen, P.; Lee, N.V.; Hu, W.; Xu, M.; Ferre, R.A.; Lam, H.; Bergqvist, S.; Solowiej, J.; Diehl, W.; He, Y.-A.; et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol. Cancer Ther. 2016, 15, 2273–2281. [Google Scholar] [CrossRef]
- Kim, S.; Tiedt, R.; Loo, A.; Horn, T.; Delach, S.; Kovats, S.; Haas, K.; Engstler, B.S.; Cao, A.; Pinzon-Ortiz, M.; et al. The Potent and Selective Cyclin-Dependent Kinases 4 and 6 Inhibitor Ribociclib (LEE011) Is a Versatile Combination Partner in Preclinical Cancer Models. Oncotarget 2018, 9, 35226–35240. [Google Scholar] [CrossRef]
- Sammons, S.L.; Topping, D.L.; Blackwell, K.L. HR+, HER2– Advanced Breast Cancer and CDK4/6 Inhibitors: Mode of Action, Clinical Activity, and Safety Profiles. Curr. Cancer Drug Targets 2017, 17, 637–649. [Google Scholar] [CrossRef]
- Zhang, Z.; Golomb, L.; Meyerson, M. Functional Genomic Analysis of CDK4 and CDK6 Gene Dependency across Human Cancer Cell Lines. Cancer Res. 2022, 82, 2171–2184. [Google Scholar] [CrossRef]
- Azim, H.A.; Ghosn, M.; Oualla, K.; Kassem, L. Personalized Treatment in Metastatic Triple-negative Breast Cancer: The Outlook in 2020. Breast J. 2020, 26, 69–80. [Google Scholar] [CrossRef]
- Wells, C.I.; Vasta, J.D.; Corona, C.R.; Wilkinson, J.; Zimprich, C.A.; Ingold, M.R.; Pickett, J.E.; Drewry, D.H.; Pugh, K.M.; Schwinn, M.K.; et al. Quantifying CDK Inhibitor Selectivity in Live Cells. Nat. Commun. 2020, 11, 2743. [Google Scholar] [CrossRef] [PubMed]
- George, M.A.; Qureshi, S.; Omene, C.; Toppmeyer, D.L.; Ganesan, S. Clinical and Pharmacologic Differences of CDK4/6 Inhibitors in Breast Cancer. Front. Oncol. 2021, 11, 693104. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; Demichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- ESMO-Magnitude of Clinical Benefit Scale. Available online: www.esmo.org/guidelines/esmo-mcbs (accessed on 20 February 2023).
- National Comprehensive Care Network. Clinical Practice Guidelines in Oncology Breast Cancer v3.2023. Available online: https://NCCN.org (accessed on 20 February 2023).
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer. JAMA Oncol. 2016, 2, 1310. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Kilburn, L.; Fribbens, C.; Beaney, M.; Garcia-Murillas, I.; Budzar, A.U.; Robertson, J.F.R.; Gradishar, W.; Piccart, M.; et al. ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor–Positive Breast Cancer: A Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin. Cancer Res. 2020, 26, 5172–5177. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Eng. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Buzdar, A.; Douma, J.; Davidson, N.; Elledge, R.; Morgan, M.; Smith, R.; Porter, L.; Nabholtz, J.; Xiang, X.; Brady, C. Phase III, Multicenter, Double-Blind, Randomized Study of Letrozole, an Aromatase Inhibitor, for Advanced Breast Cancer Versus Megestrol Acetate. J. Clin. Oncol. 2001, 19, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U.; Jonat, W.; Howell, A.; Jones, S.E.; Blomqvist, C.P.; Vogel, C.L.; Eiermann, W.; Wolter, J.M.; Steinberg, M.; Webster, A.; et al. Anastrozole versus Megestrol Acetate in the Treatment of Postmenopausal Women with Advanced Breast Carcinoma: Results of a Survival Update Based on a Combined Analysis of Data from Two Mature Phase III Trials. Arimidex Study Group. Cancer 1998, 83, 1142–1152. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Noguchi, S.; Shao, Z.; Grinsted, L.M.; Fazal, M.; Ellis, M.J. Abstract P2-08-02: Progression-Free Survival Results in Patient Subgroups from a Phase 3 Randomized Trial of Fulvestrant 500 Mg vs Anastrozole for Hormone Receptor-Positive Advanced Breast Cancer (FALCON). Cancer Res. 2017, 77 (Suppl. S4), P2-08-02. [Google Scholar] [CrossRef]
- Eli Lilly Canada Inc. Verzenio Product Monograph; Eli Lilly Canada Inc.: Toronto, ON, Canada, 2023. [Google Scholar]
- Pfizer Canada ULC. Ibrance Product Monograph; Pfizer Canada ULC: Kirkland, QC, Canada, 2023. [Google Scholar]
- Novartis Pharmaceuticals Canada Inc. Kisqali Product Monograph; Novartis Pharmaceuticals Canada Inc.: Montreal, QC, Canada, 2023. [Google Scholar]
- Bidard, F.-C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; et al. Switch to Fulvestrant and Palbociclib versus No Switch in Advanced Breast Cancer with Rising ESR1 Mutation during Aromatase Inhibitor and Palbociclib Therapy (PADA-1): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef]
- Henry, N.L.; Somerfield, M.R.; Dayao, Z.; Elias, A.; Kalinsky, K.; McShane, L.M.; Moy, B.; Park, B.H.; Shanahan, K.M.; Sharma, P.; et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 3205–3221. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events; 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 20 February 2023).
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the Task Force on Cardio-Oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Thein, K.Z.; Ball, S.; Swarup, S.; Sultan, A.; Zaw, M.H.; Tijani, L.; Awasthi, S.; Hardwicke, F.; Jones, C. CLO19-052: Incidence of Ribociclib-Associated Cardiac Conduction Abnormalities in Patients with Hormone Receptor-Positive HER2-Negative Metastatic Breast Cancer: A Combined Analysis of 3 Phase III Randomized Controlled Trials. J. Natl. Compr. Canc. Netw. 2019, 17, CLO19-052. [Google Scholar] [CrossRef]
- Chin, Y.M.; Shibayama, T.; Chan, H.T.; Otaki, M.; Hara, F.; Kobayashi, T.; Kobayashi, K.; Hosonaga, M.; Fukada, I.; Inagaki, L.; et al. Serial Circulating Tumor DNA Monitoring of CDK4/6 Inhibitors Response in Metastatic Breast Cancer. Cancer Sci. 2022, 113, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting Chemotherapy Toxicity in Older Adults with Cancer: A Prospective Multicenter Study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Saliba, D.; Elliott, M.; Rubenstein, L.Z.; Solomon, D.H.; Young, R.T.; Kamberg, C.J.; Roth, C.; MacLean, C.H.; Shekelle, P.G.; Sloss, E.M.; et al. The Vulnerable Elders Survey: A Tool for Identifying Vulnerable Older People in the Community. J. Am. Geriatr. Soc. 2001, 49, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Torres, C.; Hsu, T. Comprehensive Geriatric Assessment in the Older Adult with Cancer: A Review. Eur. Urol. Focus 2017, 3, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Decoster, L.; Van Puyvelde, K.; Mohile, S.; Wedding, U.; Basso, U.; Colloca, G.; Rostoft, S.; Overcash, J.; Wildiers, H.; Steer, C.; et al. Screening Tools for Multidimensional Health Problems Warranting a Geriatric Assessment in Older Cancer Patients: An Update on SIOG Recommendations. Ann. Oncol. 2015, 26, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Drubbel, I.; Bleijenberg, N.; Kranenburg, G.; Eijkemans, R.J.C.; Schuurmans, M.J.; de Wit, N.J.; Numans, M.E. Identifying Frailty: Do the Frailty Index and Groningen Frailty Indicator Cover Different Clinical Perspectives? A Cross-Sectional Study. BMC Fam. Pract. 2013, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 254–259. [Google Scholar] [CrossRef]
- Ostwal, V.; Ramaswamy, A.; Bhargava, P.; Hatkhambkar, T.; Swami, R.; Rastogi, S.; Mandavkar, S.; Ghosh, J.; Bajpai, J.; Gulia, S.; et al. Cancer Aging Research Group (CARG) Score in Older Adults Undergoing Curative Intent Chemotherapy: A Prospective Cohort Study. BMJ Open 2021, 11, e047376. [Google Scholar] [CrossRef]
- Cardoso, F.; Jacot, W.; Kummel, S.; Gupta, S.; Balaraman, R.; Lebedeva, L.; Ji, Y.; Lakshmanan, A.; Amin, K.; Li, Z.; et al. Primary Efficacy and Safety Results from the AMALEE Trial Evaluating 600mg vs 400mg Starting Doses of First-Line Ribociclib in Patients with HR+/HER2- Advanced Breast Cancer. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Ettl, J.; Im, S.-A.; Ro, J.; Masuda, N.; Colleoni, M.; Schnell, P.; Bananis, E.; Lu, D.R.; Cristofanilli, M.; Rugo, H.S.; et al. Hematologic Adverse Events Following Palbociclib Dose Reduction in Patients with Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Pooled Analysis from Randomized Phase 2 and 3 Studies. Breast Cancer Res. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Moftakhar, B.; Lekkala, M.; Strawderman, M.; Smith, T.C.; Meacham, P.; Fitzgerald, B.; Falkson, C.I.; Dhakal, A. Impact of Early Dose Intensity Reduction of Palbociclib on Clinical Outcomes in Patients with Hormone-Receptor-Positive Metastatic Breast Cancer. Breast Cancer Res. Treat. 2020, 183, 411–418. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine Treatment versus Chemotherapy in Postmenopausal Women with Hormone Receptor-Positive, HER2-Negative, Metastatic Breast Cancer: A Systematic Review and Network Meta-Analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- SABCS 2022: Primary Results from the Randomized Phase II RIGHT Choice Trial of Premenopausal Patients with Aggressive HR+/HER2− Advanced Breast Cancer Treated with Ribociclib + Endocrine Therapy vs Physician’s Choice Combination Chemotherapy. Available online: https://clin.larvol.com/abstract-detail/SABCS%202022/60535039 (accessed on 9 March 2023).
- Michaud, K.; Solomon, D.A.; Oermann, E.; Kim, J.-S.; Zhong, W.-Z.; Prados, M.D.; Ozawa, T.; James, C.D.; Waldman, T. Pharmacologic Inhibition of Cdk4/6 Arrests the Growth of Glioblastoma Multiforme Intracranial Xenografts. Cancer Res. 2010, 70, 3228–3238. [Google Scholar] [CrossRef]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical Characterization of the CDK4/6 Inhibitor LY2835219: In-Vivo Cell Cycle-Dependent/Independent Anti-Tumor Activities Alone/in Combination with Gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef]
- Watase, C.; Shiino, S.; Shimoi, T.; Noguchi, E.; Kaneda, T.; Yamamoto, Y.; Yonemori, K.; Takayama, S.; Suto, A. Breast Cancer Brain Metastasis—Overview of Disease State, Treatment Options and Future Perspectives. Cancers 2021, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor-Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef] [PubMed]
- Barberi, V.; Pietragalla, A.; Franceschini, G.; Marazzi, F.; Paris, I.; Cognetti, F.; Masetti, R.; Scambia, G.; Fabi, A. Oligometastatic Breast Cancer: How to Manage It? J. Pers. Med. 2021, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Network, C.B.C. Breast Cancer—The Lived Experience. Available online: https://cbcn.ca/web/default/files/public/Reports/FINAL%20ENG%20Lived%20Experience%20Report-compressed.pdf (accessed on 20 February 2023).
- Holle, L.M.; Bilse, T.; Alabelewe, R.M.; Kintzel, P.E.; Kandemir, E.A.; Tan, C.J.; Weru, I.; Chambers, C.R.; Dobish, R.; Handel, E.; et al. International Society of Oncology Pharmacy Practitioners (ISOPP) Position Statement: Role of the Oncology Pharmacy Team in Cancer Care. J. Oncol. Pharm. Pract. 2021, 27, 785–801. [Google Scholar] [CrossRef]
- Howell, D.; Keshavarz, H.; Broadfield, L.; Hack, T.; Hamel, M.; Harth, T.; Jones, J.; McLeod, D.; Olson, K.; Phan, S.; et al. A Pan Canadian Practice Guideline for Screening, Assessment, and Management of Cancer-Related Fatigue in Adults Version 2; Canadian Partnership Against Cancer (Cancer Journey Advisory Group) and the Canadian Association of Psychosocial Oncology: Edmonton, AB, Canada, 2015. [Google Scholar] [CrossRef]









| ABC 5 * [30] | ESMO 2021 * [31] | NCCN (Version 3.2023) [33] |
|---|---|---|
| CDK4/6i combined with ET (AI or FUL) ESMO-MCBS v1.1 † scores:
| CDK4/6i combined with ET (AI or FUL):
| AI + CDK4/6i
|
| Abemaciclib [41] | Palbociclib [42] | Ribociclib [43] | |
|---|---|---|---|
| CBC | Prior to start Q2W for months 1, 2 QM for months 3, 4 As clinically indicated | Prior to start Day 15 for cycle 1, 2 As clinically indicated | Prior to start Q2W for cycles 1, 2 At the start of cycles 3–6 As clinically indicated |
| Liver function | Prior to start Q2W for months 1,2 QM for months 3,4 As clinically indicated | Monitor for signs of hepatoxicity | Prior to start Q2W for cycles 1, 2 At the start of cycles 3–6 As clinically indicated |
| ILD/pneumonitis | Monitor for pulmonary symptoms indicative of ILD/pneumonitis (hypoxia, cough, dyspnea) | ||
| ECG | – | – | Prior to start Day 14 of cycle 1 Beginning of cycle 2 At regular intervals during steady state (approx. cycle day 14) & as clinically indicated |
| Electrolytes | – | – | Prior to start At regular intervals & as clinically indicated |
| Infection or myelosuppression | Monitor for signs and symptoms | ||
| Thrombo-embolism | Monitor for signs and symptoms | – | Monitor for signs and symptoms in patients at risk |
| Tool | Format | Clinical Utility |
|---|---|---|
| CARG [57] | Physician-administered; assesses 11 clinical parameters | Used to assess toxicity risk in geriatric patients receiving chemotherapy |
| VES-13 [52] | Patient-reported; 13-item function-based assessment | Detects vulnerable older adults who are at an increased risk of death or functional decline over the subsequent 2 years |
| CGA [53] | Physician-administered; assesses functional status, comorbidities, polypharmacy, cognition, psychological status, social support and nutrition | Used to identify changes that are potentially treatable to improve patient outcomes |
| Geriatric 8 [54] | Physician-administered; 8-item screening tool | Specific to cancer patients; rapid (5 min) |
| Groningen [55] | Patient-reported; 15-item self-assessment | Reflects current problems in a patient’s daily life |
| Clinical Frailty Scale [56] | Physician-administered; based on observation and clinical judgement (not a questionnaire) | Not specific to oncology; requires clinical judgement |
| Study | Subgroup | PFS HR (95% CI) |
|---|---|---|
| PALOMA-2 (49% visceral) | Site of metastases at baseline | |
| Visceral | 0.63 (0.47–0.85) | |
| Nonvisceral | 0.50 (0.36–0.70) | |
| MONALEESA-2 (59% visceral) | Liver or lung metastases | |
| Yes | 0.561 (0.424–0.743) | |
| No | 0.597 (0.426–0.837) | |
| MONARCH-3 (53% visceral) | Metastatic site | |
| Visceral | 0.61 (0.42–0.87) | |
| Bone only | 0.58 (0.27–1.25) | |
| Other | 0.34 (0.19–0.61) | |
| MONALEESA-7 (57% visceral) | Liver or lung metastases | |
| Yes | 0.50 (0.38–0.68) | |
| No | 0.64 (0.45–0.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzak, K.J.; Bouganim, N.; Brezden-Masley, C.; Edwards, S.; Gelmon, K.; Henning, J.-W.; Hilton, J.F.; Sehdev, S. HR+/HER2– Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review. Curr. Oncol. 2023, 30, 5425-5447. https://doi.org/10.3390/curroncol30060411
Jerzak KJ, Bouganim N, Brezden-Masley C, Edwards S, Gelmon K, Henning J-W, Hilton JF, Sehdev S. HR+/HER2– Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review. Current Oncology. 2023; 30(6):5425-5447. https://doi.org/10.3390/curroncol30060411
Chicago/Turabian StyleJerzak, Katarzyna J., Nathaniel Bouganim, Christine Brezden-Masley, Scott Edwards, Karen Gelmon, Jan-Willem Henning, John F. Hilton, and Sandeep Sehdev. 2023. "HR+/HER2– Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review" Current Oncology 30, no. 6: 5425-5447. https://doi.org/10.3390/curroncol30060411
APA StyleJerzak, K. J., Bouganim, N., Brezden-Masley, C., Edwards, S., Gelmon, K., Henning, J.-W., Hilton, J. F., & Sehdev, S. (2023). HR+/HER2– Advanced Breast Cancer Treatment in the First-Line Setting: Expert Review. Current Oncology, 30(6), 5425-5447. https://doi.org/10.3390/curroncol30060411