Leptomeningeal Metastasis: A Review of the Pathophysiology, Diagnostic Methodology, and Therapeutic Landscape
Abstract
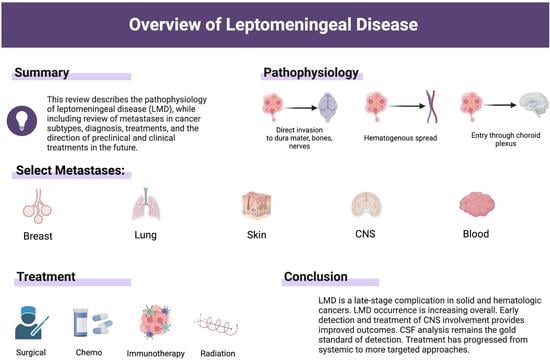
:1. Introduction: The Pathophysiology of Leptomeningeal Disease
2. Breast Cancer
3. Lung Cancer
4. Melanoma
5. Primary Central Nervous System Tumors
6. Hematologic Cancers
7. Leptomeningeal Mimics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMD | Leptomeningeal disease |
| MS | Multiple sclerosis |
| CNS | Central nervous system |
| NHL | Non-Hodgkin’s lymphoma |
| ALL | Acute lymphoblastic leukemia |
| CSF | Cerebrospinal fluid |
| CN | Cranial nerve |
| NCNN | National Comprehensive Cancer Network |
| KPS | Karnofsky performance scale |
| SWS | Sturge-Weber syndrome |
| MRI | Magnetic resonance imaging |
| CEA | Carcinoembryonic antigen |
| ICP | Intracranial pressure |
| VPS | Ventriculoperitoneal shunt |
| WBRT | Whole brain radiation therapy |
| CSI | Craniospinal irradiation |
| ICI | Immune checkpoint inhibitor |
| RCT | Randomized controlled trial |
| MC | Meningeal carcinomatosis |
| LBM | Leptomeningeal breast cancer metastasis |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| EGFR | Epidermal growth factor receptor |
| BBB | Blood brain barrier |
| ctDNA | Tumor derived DNA |
| HCB | Hot cross bun |
| LLM | Leptomeningeal lung cancer metastasis |
| NSCLC | Non-small cell lung cancer |
| BM | Brain metastases |
| ALK | Anaplastic lymphoma kinase |
| SCLC | Small cell lung carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| ACTA2 | Actin gene |
| EpCAMs | Epithelial cell adhesion molecules |
| TKI | Tyrosine kinase inhibitor |
| LMM | leptomeningeal melanoma metastasis |
| MCAM | Melanoma cell adhesion molecule |
| MMP | Metalloproteinase |
| VEGFA | Vascular endothelial growth factor A |
| CTC | Circulating tumor cells |
| NGS | Next Generation Sequencing |
| ddPCR | Droplet digital PCR |
| TT | Targeted therapies |
| GBM | Glioblastoma |
| CML | Chronic myelogenous leukemia |
| CLL | Chronic lymphoblastic leukemia |
| GCO | Global cancer observatory |
| SEER | Surveillance, epidemiology, and end results |
| CBC | Complete blood count |
| WBC | White blood count |
| HMA | Hypomethylating agent |
| FLT3 | FMS-like tyrosine kinase 3 |
| BCMA | B-cell maturation antigen |
| ACE | Angiotensin converting enzyme |
References
- Nayar, G.; Ejikeme, T.; Chongsathidkiet, P.; Elsamadicy, A.A.; Blackwell, K.L.; Clarke, J.M.; Lad, S.P.; Fecci, P.E. Leptomeningeal disease: Current diagnostic and therapeutic strategies. Oncotarget 2017, 8, 73312–73328. [Google Scholar] [CrossRef] [Green Version]
- Gleissner, B.; Chamberlain, M.C. Neoplastic meningitis. Lancet Neurol. 2006, 5, 443–452. [Google Scholar] [CrossRef]
- Batool, A.; Kasi, A. Leptomeningeal Carcinomatosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nguyen, T.K.; Nguyen, E.K.; Soliman, H. An overview of leptomeningeal disease. Ann. Palliat. Med. 2021, 10, 909–922. [Google Scholar] [CrossRef]
- Horbinski, C.; Nabors, L.B.; Portnow, J.; Baehring, J.; Bhatia, A.; Bloch, O.; Brem, S.; Butowski, N.; Cannon, D.M.; Chao, S.; et al. NCCN Guidelines® Insights: Central Nervous System Cancers, Version 2.2022. J. Natl. Compr. Cancer Netw. 2023, 21, 12–20. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Turner, B.E.; Asher, A.L.; Marcrom, S.R.; Fiveash, J.B.; Foreman, P.M.; Press, R.H.; Patel, K.R.; Curran, W.J.; Breen, W.G.; et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro-Oncology 2019, 21, 1049–1059. [Google Scholar] [CrossRef]
- Collie, D.; Brush, J.; Lammie, G.; Grant, R.; Kunkler, I.; Leonard, R.; Gregor, A.; Sellar, R. Imaging features of leptomeningeal metastases. Clin. Radiol. 1999, 54, 765–771. [Google Scholar] [CrossRef]
- Pavlidis, N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann. Oncol. 2004, 15 (Suppl. S4), iv285–iv291. [Google Scholar] [CrossRef]
- van Bussel, M.T.J.; Pluim, D.; Milojkovic Kerklaan, B.; Bol, M.; Sikorska, K.; Linders, D.T.; Broek, D.V.D.; Beijnen, J.H.; Schellens, J.H.; Brandsma, D. Circulating epithelial tumor cell analysis in CSF in patients with leptomeningeal metastases. Neurology 2020, 94, e521–e528. [Google Scholar] [CrossRef]
- Nayak, L.; Fleisher, M.; Gonzalez-Espinoza, R.; Lin, O.; Panageas, K.; Reiner, A.; Liu, C.-M.; DeAngelis, L.M.; Omuro, A. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology 2013, 80, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Piña, Y.; Yadugiri, S.; Yeboa, D.N.; Ferguson, S.D.; Forsyth, P.A.; Oliva, I.C.G. Advances in Diagnosis and Treatment for Leptomeningeal Disease in Melanoma. Curr. Oncol. Rep. 2022, 24, 43–54. [Google Scholar] [CrossRef]
- Gunther, J.R.; Rahman, A.R.; Dong, W.; Yehia, Z.A.; Kebriaei, P.; Rondon, G.; Pinnix, C.; Milgrom, S.A.; Allen, P.K.; Dabaja, B.S.; et al. Craniospinal irradiation prior to stem cell transplant for hematologic malignancies with CNS involvement: Effectiveness and toxicity after photon or proton treatment. Pract. Radiat. Oncol. 2017, 7, e401–e408. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Bot, I.; Blank, C.U.; Brandsma, D. Clinical and radiological response of leptomeningeal melanoma after whole brain radiotherapy and ipilimumab. J. Neurol. 2012, 259, 1976–1978. [Google Scholar] [CrossRef]
- Wu, R.C.; Newman, W.; Patanowitz, L.; Branstetter, B.F.; Amankulor, N.; Tarhini, A.A. Long-term control of leptomeningeal disease after radiation therapy and nivolumab in a metastatic melanoma patient. Immunotherapy 2020, 12, 763–769. [Google Scholar] [CrossRef]
- Glitza, I.C.; Bucheit, A.D. Clinical response of central nervous system melanoma to anti-PD1 therapy in 2 melanoma patients. Arch. Immunol. 2017, 1, 1–3. [Google Scholar]
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, Based on 2021 Submission Data (1999–2019); U.S. Department of Health and Human Services: Washington, DC, USA, 2023. [Google Scholar]
- Alnajar, H.; Rosen, L.; Javidiparsijani, S.; Al-Ghamdi, Y.; Gattuso, P. Prognostic Markers and Histologic Subtypes in Patients with Meningeal Carcinomatosis in Breast Cancer. Acta Cytol. 2017, 61, 140–144. [Google Scholar] [CrossRef]
- Quigley, M.R.; Fukui, O.; Chew, B.; Bhatia, S.; Karlovits, S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg. Rev. 2012, 36, 377–382. [Google Scholar] [CrossRef]
- Watanabe, J.; Mitsuya, K.; Nakamoto, S.; Harada, H.; Deguchi, S.; Hayashi, N.; Nakasu, Y. Leptomeningeal Metastasis in ER + HER2- Advanced Breast Cancer Patients: A Review of the Cases in a Single Institute Over a 15-year Period. Breast Cancer Res. Treat. 2021, 189, 225–236. [Google Scholar] [CrossRef]
- Okada, Y.; Abe, T.; Shinozaki, M.; Tanaka, A.; Kobayashi, M.; Gomi, H.; Kanemaki, Y.; Nakamura, N.; Kojima, Y. Evaluation of imaging findings and prognostic factors after whole-brain radiotherapy for carcinomatous meningitis from breast cancer: A retrospective analysis. Medicine 2020, 99, e21333. [Google Scholar] [CrossRef]
- de Azevedo, C.R.A.S.; Cruz, M.R.S.; Chinen, L.T.D.; Peres, S.V.; Peterlevitz, M.A.; Pereira, A.E.D.A.; Fanelli, M.F.; Gimenes, D.L. Meningeal carcinomatosis in breast cancer: Prognostic factors and outcome. J. Neuro-Oncol. 2011, 104, 565–572. [Google Scholar] [CrossRef]
- Gauthier, H.; Guilhaume, M.N.; Bidard, F.C.; Pierga, J.Y.; Girre, V.; Cottu, P.H.; Laurence, V.; Livartowski, A.; Mignot, L.; Diéras, V. Survival of breast cancer patients with meningeal carcinomatosis. Ann. Oncol. 2010, 21, 2183–2187. [Google Scholar] [CrossRef]
- Park, I.H.; Kwon, Y.; Ro, J.Y.; Lee, K.S.; Ro, J. Concordant HER2 status between metastatic breast cancer cells in CSF and primary breast cancer tissue. Breast Cancer Res. Treat. 2009, 123, 125–128. [Google Scholar] [CrossRef]
- Tewarie, I.A.; Jessurun, C.A.C.; Hulsbergen, A.F.C.; Smith, T.R.; Mekary, R.A.; Broekman, M.L.D. Leptomeningeal disease in neurosurgical brain metastases patients: A systematic review and meta-analysis. Neuro-Oncol. Adv. 2021, 3, vdab162. [Google Scholar] [CrossRef]
- Hyun, J.-W.; Jeong, I.H.; Joung, A.; Cho, H.J.; Kim, S.-H.; Kim, H.J. Leptomeningeal metastasis: Clinical experience of 519 cases. Eur. J. Cancer 2016, 56, 107–114. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Melisko, M.; Roy, R.; Sosa, E.V.; Hauranieh, L.; Kablanian, A.; Eisenbud, L.E.; Ryazantsev, A.; Au, A.; Scott, J.H.; et al. Molecular Profiling of Tumor Cells in Cerebrospinal Fluid and Matched Primary Tumors from Metastatic Breast Cancer Patients with Leptomeningeal Carcinomatosis. Cancer Res 2013, 73, 7134–7143. [Google Scholar] [CrossRef] [Green Version]
- Orrantia-Borunda, E.; Anchondo-Nunez, P.; Acuna-Aguilar, L.E.; Gomez-Valles, F.O.; Ramirez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Miller, L.; Metheny-Barlow, L.; Lo, H.-W. EGFR and HER2 signaling in breast cancer brain metastasis. Front. Biosci. 2016, 8, 245–263. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.-C.; Zhang, L.; Zhang, C.; Lowery, F.J.; Ding, Z.; Guo, H.; Wang, H.; Huang, S.; Sahin, A.A.; et al. Src Family Kinases as Novel Therapeutic Targets to Treat Breast Cancer Brain Metastases. Cancer Res. 2013, 73, 5764–5774. [Google Scholar] [CrossRef] [Green Version]
- Momeny, M.; Saunus, J.M.; Marturana, F.; Reed, A.E.M.; Black, D.; Sala, G.; Iacobelli, S.; Holland, J.D.; Yu, D.; Da Silva, L.; et al. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget 2015, 6, 3932–3946. [Google Scholar] [CrossRef] [Green Version]
- Priedigkeit, N.; Hartmaier, R.J.; Chen, Y.; Vareslija, D.; Basudan, A.; Watters, R.J.; Thomas, R.; Leone, J.P.; Lucas, P.C.; Bhargava, R.; et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol. 2017, 3, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.D.; Avkshtol, V.; Baschnagel, A.M.; Meyer, K.; Ye, H.; Grills, I.S.; Chen, P.Y.; Maitz, A.; Olson, R.E.; Pieper, D.R.; et al. Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated With Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2015, 94, 537–543. [Google Scholar] [CrossRef]
- Jung, J.-M.; Kim, S.; Joo, J.; Shin, K.H.; Gwak, H.-S.; Lee, S.H. Incidence and Risk Factors for Leptomeningeal Carcinomatosis in Breast Cancer Patients with Parenchymal Brain Metastases. J. Korean Neurosurg. Soc. 2012, 52, 193–199. [Google Scholar] [CrossRef]
- Mills, M.N.; King, W.; Soyano, A.; Pina, Y.; Czerniecki, B.J.; Forsyth, P.A.; Soliman, H.; Han, H.S.; Ahmed, K.A. Evolving management of HER2+ breast cancer brain metastases and leptomeningeal disease. J. Neuro-Oncol. 2022, 157, 249–269. [Google Scholar] [CrossRef]
- Glass, J.P.; Melamed, M.; Chernik, N.L.; Posner, J.B. Malignant cells in cerebrospinal fluid (CSF): The meaning of a positive CSF cytology. Neurology 1979, 29, 1369–1375. [Google Scholar] [CrossRef] [Green Version]
- Twijnstra, A.; van Zanten, A.P.; Nooyen, W.J.; Ongerboer de Visser, B.W. Sensitivity and specificity of single and combined tumour markers in the diagnosis of leptomeningeal metastasis from breast cancer. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Malani, R.; Fleisher, M.; Kumthekar, P.; Lin, X.; Omuro, A.; Groves, M.D.; Lin, N.U.; Melisko, M.; Lassman, A.B.; Jeyapalan, S.; et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J. Neuro-Oncol. 2020, 148, 599–606. [Google Scholar] [CrossRef]
- Torre, M.; Lee, E.Q.; Chukwueke, U.N.; Nayak, L.; Cibas, E.S.; Lowe, A.C. Integration of rare cell capture technology into cytologic evaluation of cerebrospinal fluid specimens from patients with solid tumors and suspected leptomeningeal metastasis. J. Am. Soc. Cytopathol. 2019, 9, 45–54. [Google Scholar] [CrossRef]
- Angus, L.; Deger, T.; Jager, A.; Martens, J.W.; de Weerd, V.; van Heuvel, I.; Bent, M.J.v.D.; Smitt, P.A.S.; Kros, J.M.; Bindels, E.M.; et al. Detection of Aneuploidy in Cerebrospinal Fluid from Patients with Breast Cancer Can Improve Diagnosis of Leptomeningeal Metastases. Clin. Cancer Res. 2021, 27, 2798–2806. [Google Scholar] [CrossRef]
- Fitzpatrick, A.; Iravani, M.; Mills, A.; Childs, L.; Alaguthurai, T.; Clifford, A.; Garcia-Murillas, I.; Van Laere, S.; Dirix, L.; Harries, M.; et al. Assessing CSF ctDNA to Improve Diagnostic Accuracy and Therapeutic Monitoring in Breast Cancer Leptomeningeal Metastasis. Clin. Cancer Res. 2022, 28, 1180–1191. [Google Scholar] [CrossRef]
- Morikawa, A.; Jordan, L.; Rozner, R.; Patil, S.; Boire, A.; Pentsova, E.; Seidman, A.D. Characteristics and Outcomes of Patients With Breast Cancer With Leptomeningeal Metastasis. Clin. Breast Cancer 2016, 17, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Niwińska, A.; Pogoda, K.; Michalski, W.; Kunkiel, M.; Jagiełło-Gruszfeld, A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J. Neuro-Oncol. 2018, 138, 191–198. [Google Scholar] [CrossRef]
- Carausu, M.; Carton, M.; Darlix, A.; Pasquier, D.; Leheurteur, M.; Debled, M.; Mouret-Reynier, M.; Goncalves, A.; Dalenc, F.; Verret, B.; et al. Breast cancer patients treated with intrathecal therapy for leptomeningeal metastases in a large real-life database. ESMO Open 2021, 6, 100150. [Google Scholar] [CrossRef]
- Le Rhun, E.; Wallet, J.; Mailliez, A.; Le Deley, M.C.; Rodrigues, I.; Boulanger, T.; Lorgis, V.; Barrière, J.; Robin, Y.M.; Weller, M.; et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro-Oncology 2020, 22, 524–538. [Google Scholar] [CrossRef] [Green Version]
- Oberkampf, F.; Gutierrez, M.; Trabelsi Grati, O.; Rhun, É.L.; Trédan, O.; Turbiez, I.; Kadi, A.; Dubot, C.; Taillibert, S.; Vacher, S.; et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro-Oncology 2022, 25, 365–374. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non–Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563–570. [Google Scholar] [CrossRef]
- Pellerino, A.; Bruno, F.; Rudà, R.; Soffietti, R. Systemic Therapy for Lung Cancer Brain Metastases. Curr. Treat. Options Oncol. 2021, 22, 110. [Google Scholar] [CrossRef]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro-Oncology 2021, 23, 1447–1456. [Google Scholar] [CrossRef]
- Turkaj, A.; Morelli, A.M.; Vavalà, T.; Novello, S. Management of Leptomeningeal Metastases in Non-oncogene Addicted Non-small Cell Lung Cancer. Front. Oncol. 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lin, Z.; Hong, Y.; Fu, Y.; Chen, Y.; Liu, T.; Zheng, Y.; Tian, J.; Liu, C.; Pu, W.; et al. Brain parenchymal and leptomeningeal metastasis in non-small cell lung cancer. Sci. Rep. 2022, 12, 22372. [Google Scholar] [CrossRef]
- Naidoo, J.; Schreck, K.C.; Fu, W.; Hu, C.; Carvajal-Gonzalez, A.; Connolly, R.M.; Santa-Maria, C.A.; Lipson, E.J.; Holdhoff, M.; Forde, P.M.; et al. Pembrolizumab for patients with leptomeningeal metastasis from solid tumors: Efficacy, safety, and cerebrospinal fluid biomarkers. J. Immunother. Cancer 2021, 9, e002473. [Google Scholar] [CrossRef]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Mitra, D.; Chen, Y.-H.; Li, R.; Hermann, G.; Atkins, K.; Kozono, D.; Baldini, E.H.; Aizer, A.; Chukwueke, U.; Mak, R.H. EGFR mutant locally advanced non-small cell lung cancer is at increased risk of brain metastasis. Clin. Transl. Radiat. Oncol. 2019, 18, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; Riely, G.J.; et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016, 17, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, G.; Singh, M.; Vredenburgh, J.J. Leptomeningeal Metastasis from Non–Small Cell Lung Cancer and Current Landscape of Treatments. Clin. Cancer Res. 2022, 29, 11–29. [Google Scholar] [CrossRef]
- Higo, H.; Igawa, T.; Matsuoka, K.; Kawaji, H.; Suzaki, N.; Nagata, T.; Nagayama, M.; Marukawa, M. Invasion of small cell lung cancer into the limbic system from leptomeningeal metastases. Respir. Med. Case Rep. 2021, 33, 101417. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Wen, Y.; Zhou, C. Non-small cell lung cancer in China. Cancer Commun. 2022, 42, 937–970. [Google Scholar] [CrossRef]
- Chen, K.-C.; Tsai, S.-W.; Shie, R.-H.; Zeng, C.; Yang, H.-Y. Indoor Air Pollution Increases the Risk of Lung Cancer. Int. J. Environ. Res. Public Health 2022, 19, 1164. [Google Scholar] [CrossRef]
- Alexander, M.; Lin, E.; Cheng, H. Leptomeningeal Metastases in Non-small Cell Lung Cancer: Optimal Systemic Management in NSCLC With and Without Driver Mutations. Curr. Treat. Options Oncol. 2020, 21, 72. [Google Scholar] [CrossRef]
- Ko, J.; Winslow, M.M.; Sage, J. Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med. 2020, 13, e13122. [Google Scholar] [CrossRef]
- Niu, F.-Y.; Zhou, Q.; Yang, J.-J.; Zhong, W.-Z.; Chen, Z.-H.; Deng, W.; He, Y.-Y.; Chen, H.-J.; Zeng, Z.; Ke, E.-E.; et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer 2016, 16, 149. [Google Scholar] [CrossRef] [Green Version]
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, X.; Li, N.-J.; Xue, J.-X. Leptomeningeal metastases in non-small cell lung cancer: Diagnosis and treatment. Lung Cancer 2022, 174, 1–13. [Google Scholar] [CrossRef]
- Yokawa, K.; Matsumoto, Y.; Nagakita, K.; Shinno, Y.; Kudo, K.; Niguma, N.; Suenobu, K.; Yoshida, H. Solitary Leptomeningeal Metastasis from Lung Cancer: A Case Report. NMC Case Rep. J. 2022, 9, 323–328. [Google Scholar] [CrossRef]
- Sharma, A.; Low, J.T.; Kumthekar, P. Advances in the Diagnosis and Treatment of Leptomeningeal Disease. Curr. Neurol. Neurosci. Rep. 2022, 22, 413–425. [Google Scholar] [CrossRef]
- Li, H.; Xia, M.; Zheng, S.; Lin, Y.; Yu, T.; Xie, Y.; Shen, Y.; Liu, X.; Qian, X.; Yin, Z. Cerebrospinal fluid exosomal microRNAs as biomarkers for diagnosing or monitoring the progression of non-small cell lung cancer with leptomeningeal metastases. Biotechnol. Genet. Eng. Rev. 2023, 1–22. [Google Scholar] [CrossRef]
- Senko, C.; Gunjur, A.; Balasubramanian, A.; Gan, H.K.; Parakh, S.; Cher, L. The systemic management of central nervous system metastases and leptomeningeal disease from advanced lung, melanoma, and breast cancer with molecular drivers: An Australian perspective. Asia-Pac. J. Clin. Oncol. 2022, 18, 515–525. [Google Scholar] [CrossRef]
- Cho, B.C.; Felip, E.; Hayashi, H.; Thomas, M.; Lu, S.; Besse, B.; Sun, T.; Martinez, M.; Sethi, S.N.; Shreeve, S.M.; et al. MARIPOSA: Phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer. Future Oncol. 2022, 18, 639–647. [Google Scholar] [CrossRef]
- Yang, T.J.; Wijetunga, N.A.; Yamada, J.; Wolden, S.; Mehallow, M.; Goldman, D.A.; Zhang, Z.; Young, R.J.; Kris, M.G.; Yu, H.A.; et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro-Oncology 2020, 23, 134–143. [Google Scholar] [CrossRef]
- Remon, J.; Le Rhun, E.; Besse, B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat. Rev. 2016, 53, 128–137. [Google Scholar] [CrossRef]
- Smalley, K.S.; Fedorenko, I.V.; Kenchappa, R.S.; Sahebjam, S.; Forsyth, P.A. Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy. Int. J. Cancer 2016, 139, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Chorti, E.; Kebir, S.; Ahmed, M.S.; Keyvani, K.; Umutlu, L.; Kanaki, T.; Zaremba, A.; Reinboldt-Jockenhoefer, F.; Knispel, S.; Gratsias, E.; et al. Leptomeningeal disease from melanoma—Poor prognosis despite new therapeutic modalities. Eur. J. Cancer 2021, 148, 395–404. [Google Scholar] [CrossRef]
- Jasmine, J.O.; David, S. Metastatic Disease and the Nervous System. In Neurology and General Medicine, 6th ed.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Marinova, A.M.; Reilly, C.J.L.; Wong, V.; Weiss, S.; Olszanski, A.J. Metastatic Melanoma With Leptomeningeal Disease. J. Adv. Pract. Oncol. 2021, 12, 79–83. [Google Scholar] [CrossRef]
- Braeuer, R.R.; Watson, I.R.; Wu, C.-J.; Mobley, A.K.; Kamiya, T.; Shoshan, E.; Bar-Eli, M. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014, 27, 19–36. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Cugini, E.; Nuccetelli, M.; Terrinoni, A.; Di Raimondo, C.; Lombardo, P.; Costanza, G.; Cosio, T.; Rossi, P.; Orlandi, A.; et al. MCAM/MUC18/CD146 as a Multifaceted Warning Marker of Melanoma Progression in Liquid Biopsy. Int. J. Mol. Sci. 2021, 22, 12416. [Google Scholar] [CrossRef]
- Xie, S.; Luca, M.; Huang, S.; Gutman, M.; Reich, R.; Johnson, J.P.; Bar-Eli, M. Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res. 1997, 57, 2295–2303. [Google Scholar]
- Mills, L.; Tellez, C.; Huang, S.; Baker, C.; Mccarty, M.; Green, L.; Gudas, J.M.; Feng, X.; Bar-Eli, M. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res. 2002, 62, 5106–5114. [Google Scholar]
- Ballester, L.Y.; Glitza Oliva, I.C.; Douse, D.Y.; Chen, M.M.; Lan, C.; Haydu, L.E.; Huse, J.T.; Roy-Chowdhuri, S.; Luthra, R.; Wistuba, I.I.; et al. Evaluating Circulating Tumor DNA From the Cerebrospinal Fluid of Patients With Melanoma and Leptomeningeal Disease. J. Neuropathol. Exp. Neurol. 2018, 77, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Le Rhun, E.; Tu, Q.; De Carvalho Bittencourt, M.; Farre, I.; Mortier, L.; Cai, H.; Kohler, C.; Faure, G.C. Detection and quantification of CSF malignant cells by the CellSearch® technology in patients with melanoma leptomeningeal metastasis. Med. Oncol. 2013, 30, 538. [Google Scholar] [CrossRef]
- Glitza, I.C.; Smalley, K.S.M.; Brastianos, P.K.; Davies, M.A.; McCutcheon, I.; Liu, J.K.C.; Ahmed, K.A.; Arrington, J.A.; Evernden, B.R.; Smalley, I.; et al. Leptomeningeal disease in melanoma patients: An update to treatment, challenges, and future directions. Pigment. Cell Melanoma Res. 2020, 33, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.; Ennis, M.; Jerzak, K.J. Karnofsky Performance Status (KPS) </=60 Is Strongly Associated With Shorter Brain-Specific Progression-Free Survival Among Patients With Metastatic Breast Cancer With Brain Metastases. Front Oncol. 2022, 12, 867462. [Google Scholar] [CrossRef]
- Huppert, L.A.; Melisko, M.E.; Glastonbury, C.M.; Khanafshar, E.; Daud, A.I. Treatment of Metastatic Melanoma With Leptomeningeal Disease Using Intrathecal Immunotherapy. JCO Oncol. Pract. 2020, 16, 757–759. [Google Scholar] [CrossRef]
- Schäfer, N.; Scheffler, B.; Stuplich, M.; Schaub, C.; Kebir, S.; Rehkämper, C.; Mack, F.; Niehusmann, P.; Simon, M.; Greschus, S.; et al. Vemurafenib for Leptomeningeal Melanomatosis. J. Clin. Oncol. 2013, 31, e173–e174. [Google Scholar] [CrossRef]
- Lee, J.M.; Mehta, U.N.; Dsouza, L.H.; Guadagnolo, B.A.; Sanders, D.L.; Kim, K.B. Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: A case report. Melanoma Res. 2013, 23, 175–178. [Google Scholar] [CrossRef]
- Floudas, C.S.; Chandra, A.B.; Xu, Y. Vemurafenib in leptomeningeal carcinomatosis from melanoma: A case report of near-complete response and prolonged survival. Melanoma Res. 2016, 26, 312–315. [Google Scholar] [CrossRef]
- Bander, E.D.; Yuan, M.; Carnevale, J.A.; Reiner, A.S.; Panageas, K.S.; Postow, M.A.; Tabar, V.; Moss, N.S. Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer 2021, 127, 2062–2073. [Google Scholar] [CrossRef]
- Glitza, I.C.; Ferguson, S.D.; Guha-Thakurta, N. Rapid resolution of leptomeningeal disease with targeted therapy in a metastatic melanoma patient. J. Neuro-Oncol. 2017, 133, 663–665. [Google Scholar] [CrossRef]
- Ho, C.-Y.; VandenBussche, C.J.; Huppman, A.R.; Chaudhry, R.; Ali, S.Z. Cytomorphologic and clinicoradiologic analysis of primary nonhematologic central nervous system tumors with positive cerebrospinal fluid. Cancer Cytopathol. 2014, 123, 123–135. [Google Scholar] [CrossRef]
- Burger, M.C.; Zeiner, P.S.; Jahnke, K.; Wagner, M.; Mittelbronn, M.; Steinbach, J.P. Addition of Anti-Angiogenetic Therapy with Bevacizumab to Chemo- and Radiotherapy for Leptomeningeal Metastases in Primary Brain Tumors. PLoS ONE 2016, 11, e0155315. [Google Scholar] [CrossRef] [Green Version]
- Demir, M.K.; Yapıcıer, O.; Ozturk, O.C.; Aslan, M. Posterior Third Ventricular Glioblastoma with Primary Leptomeningeal Metastasis in a Child. Pediatr. Neurosurg. 2018, 53, 205–208. [Google Scholar] [CrossRef]
- Pohar, S.; Taylor, W.; Chandan, V.S.; Shah, H.; Sagerman, R.H. Primary Presentation of Glioblastoma Multiforme With Leptomeningeal Metastasis in the Absence of Previous Craniotomy: A case report. Am. J. Clin. Oncol. 2004, 27, 640–641. [Google Scholar] [CrossRef]
- Maslehaty, H.; Cordovi, S.; Hefti, M. Symptomatic spinal metastases of intracranial glioblastoma: Clinical characteristics and pathomechanism relating to GFAP expression. J. Neuro-Oncol. 2010, 101, 329–333. [Google Scholar] [CrossRef]
- Toledano Delgado, R.; Garcia, N.; Riva-Amarante, E.; Rodriguez Pascual, J.; Garcia Leal, R.; Sendra Tello, J. Spinal leptomeningeal metastasis from cerebral glioblastoma: Case report. Neurologia 2006, 21, 378–381. [Google Scholar]
- Alatakis, S.; Malham, G.M.; Thien, C. Spinal leptomeningeal metastasis from cerebral glioblastoma multiforme presenting with radicular pain: Case report and literature review. Surg. Neurol. 2001, 56, 33–37. [Google Scholar] [CrossRef]
- Shahideh, M.; Fallah, A.; Munoz, D.G.; Loch Macdonald, R. Systematic review of primary intracranial glioblastoma multiforme with symptomatic spinal metastases, with two illustrative patients. J. Clin. Neurosci. 2012, 19, 1080–1086. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamamoto, D.; Ikeda, M.; Morikawa, M.; Ueda, K.; Tanaka, K.; Sasayama, T.; Kohmura, E. Embryonal brain tumor with unknown primary lesion and massive cerebrospinal fluid dissemination: A case report. J. Clin. Neurosci. 2018, 54, 125–128. [Google Scholar] [CrossRef]
- Jenkins, N.C.; Kalra, R.R.; Dubuc, A.; Sivakumar, W.; Pedone, C.A.; Wu, X.; Taylor, M.D.; Fults, D.W. Genetic drivers of metastatic dissemination in sonic hedgehog medulloblastoma. Acta Neuropathol. Commun. 2014, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Phi, J.H.; Choi, S.A.; Lim, S.-H.; Lee, J.; Wang, K.-C.; Park, S.-H.; Kim, S.-K. ID3 contributes to cerebrospinal fluid seeding and poor prognosis in medulloblastoma. BMC Cancer 2013, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Grunder, E.; D’ambrosio, R.; Fiaschetti, G.; Abela, L.; Arcaro, A.; Zuzak, T.; Ohgaki, H.; Lv, S.-Q.; Shalaby, T.; Grotzer, M. MicroRNA-21 suppression impedes medulloblastoma cell migration. Eur. J. Cancer 2011, 47, 2479–2490. [Google Scholar] [CrossRef]
- Hsieh, P.-C.; Wu, C.-T.; Lin, K.-L.; Jaing, T.-H.; Tseng, C.-K.; Lui, T.-N.; Jung, S.-M. The clinical experience of medulloblastoma treatment and the significance of time sequence for development of leptomeningeal metastasis. Child’s Nerv. Syst. 2008, 24, 1463–1467. [Google Scholar] [CrossRef]
- Lieu, A.-S.; Wu, C.-C.; Chai, C.-Y.; Ma, Y.-C.; Su, H.-Y. Pineocytoma with malignant transformation to pineal parenchymal tumor with intermediate differentiation and leptomeningeal dissemination after subtotal tumor resection and adjuvant radiotherapy. Indian J. Pathol. Microbiol. 2023, 66, 141. [Google Scholar] [CrossRef]
- Deng, Y.; He, L.; Gao, H.; Deng, Y.; Zhang, W.M. Primary Neuroendocrine Carcinoma of Pineal Gland With Extensive Meningeal Metastasis Detected by 18F-NOTATATE PET/CT. Clin. Nucl. Med. 2022, 47, 1105–1107. [Google Scholar] [CrossRef]
- Keskin, E.; Aydin, H.A.; Bahadir, B.; Simsek, K.; Kalayci, M. Symptomatic Spinal Seeding Metastasis of a Low-grade Oligodendroglioma. J. Coll. Physicians Surg. Pak. 2022, 32, 1347–1349. [Google Scholar] [CrossRef]
- Elefante, A.; Peca, C.; Del Basso De Caro, M.; Russo, C.; Formicola, F.; Mariniello, G.; Brunetti, A.; Maiuri, F. Symptomatic spinal cord metastasis from cerebral oligodendroglioma. Neurol. Sci. 2011, 33, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Yang, G.; Wang, Y.; Yuan, T.; Gao, Y.; Dong, L. Leptomeningeal metastases from a primary central nervous system melanoma: A case report and literature review. World J. Surg. Oncol. 2014, 12, 265. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, I.; Oka, H.; Kawano, N.; Kurata, A.; Tanaka, S.; Utsuki, S.; Suzuki, S.; Ishihara, Y.; Fujii, K. Primary intracerebral malignant melanoma with leptomeningeal spread, extradural extension and lung metastasis. Clin. Neuropathol. 2001, 20, 43–45. [Google Scholar]
- Al-Habib, A.; Lach, B.; Al Khani, A. Intracerebral rhabdoid and papillary meningioma with leptomeningeal spread and rapid clinical progression. Clin. Neuropathol. 2005, 24, 1–7. [Google Scholar]
- Tseng, Y.D.; Ng, A.K. Hematologic Malignancies. Hematol. Oncol. Clin. N. Am. 2019, 34, 127–142. [Google Scholar] [CrossRef]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care 2016, 43, 661–675. [Google Scholar] [CrossRef]
- Forsthuber, T.G.; Cimbora, D.M.; Ratchford, J.N.; Katz, E.; Stüve, O. B cell-based therapies in CNS autoimmunity: Differentiating CD19 and CD20 as therapeutic targets. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418761697. [Google Scholar] [CrossRef] [Green Version]
- Matasar, M.J.; Zelenetz, A.D. Overview of Lymphoma Diagnosis and Management. Radiol. Clin. N. Am. 2008, 46, 175–198. [Google Scholar] [CrossRef]
- Allart-Vorelli, P.; Porro, B.; Baguet, F.; Michel, A.; Cousson-Gélie, F. Haematological cancer and quality of life: A systematic literature review. Blood Cancer J. 2015, 5, e305. [Google Scholar] [CrossRef] [Green Version]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef]
- Škunca, Ž. B Cell Lymphomagenesis; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef] [Green Version]
- Jamil, A.; Mukkamalla, S.K.R. Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ansell, S.M. Brentuximab vedotin. Blood 2014, 124, 3197–3200. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Umutlu, L.; Schöder, H. Functional imaging using radiomic features in assessment of lymphoma. Methods 2020, 188, 105–111. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Lynch, R.C.; Zelenetz, A.D.; Armitage, J.O.; Carson, K.R. Surveillance Imaging for Lymphoma: Pros and Cons. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e388–e395. [Google Scholar] [CrossRef]
- Taylor, J.W.; Flanagan, E.P.; O’Neill, B.P.; Siegal, T.; Omuro, A.; DeAngelis, L.; Baehring, J.; Nishikawa, R.; Pinto, F.; Chamberlain, M.; et al. Primary leptomeningeal lymphoma: International Primary CNS Lymphoma Collaborative Group report. Neurology 2013, 81, 1690–1696. [Google Scholar] [CrossRef] [Green Version]
- Nolan, C.P.; Abrey, L.E. Leptomeningeal Metastases from Leukemias and Lymphomas. Cancer Treat Res. 2005, 125, 53–69. [Google Scholar] [CrossRef]
- Nirmal, R.M. Diagnosis of malignant lymphoma—An overview. J. Oral Maxillofac. Pathol. 2020, 24, 195–199. [Google Scholar] [CrossRef]
- Dotan, E.; Aggarwal, C.; Smith, M.R. Impact of Rituximab (Rituxan) on the Treatment of B-Cell Non-Hodgkin’s Lymphoma. Pharm. Ther. 2010, 35, 148–157. [Google Scholar]
- Baraniskin, A.; Kuhnhenn, J.; Schlegel, U.; Chan, A.; Deckert, M.; Gold, R.; Maghnouj, A.; Zöllner, H.; Reinacher-Schick, A.; Schmiegel, W.; et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 2011, 117, 3140–3146. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Liu, J. Bruton’s tyrosine kinase inhibitors in the treatment of primary central nervous system lymphoma: A mini-review. Front. Oncol. 2022, 12, 1034668. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Lyengar, V.; Mukkamalla, S.K.R.; Shimanovsky, A. Leukemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Davis, A.S.; Viera, A.J.; Mead, M.D. Leukemia: An overview for primary care. Am. Fam. Physician 2014, 89, 731–738. [Google Scholar]
- Amer, E.M.; Youssef, A.F.; Romeih, M.A.; Youssef, A.A.; Khater, H.M. Role of magnetic resonance imaging in characterization of central nervous system lesions in pediatric patients with leukemia and post-treatment complications. Egypt. J. Radiol. Nucl. Med. 2020, 51, 1–14. [Google Scholar] [CrossRef]
- Deak, D.; Gorcea-Andronic, N.; Sas, V.; Teodorescu, P.; Constantinescu, C.; Iluta, S.; Pasca, S.; Hotea, I.; Turcas, C.; Moisoiu, V.; et al. A narrative review of central nervous system involvement in acute leukemias. Ann. Transl. Med. 2021, 9, 68. [Google Scholar] [CrossRef]
- Dessie, G.; Derbew Molla, M.; Shibabaw, T.; Ayelign, B. Role of Stem-Cell Transplantation in Leukemia Treatment. Stem Cells Cloning: Adv. Appl. 2020, 13, 67–77. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Kadia, T.M.; DiNardo, C.D.; Welch, M.A.; Ravandi, F. Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach. Cancer 2021, 127, 1186–1207. [Google Scholar] [CrossRef]
- Perini, G.F.; Ribeiro, G.N.; Pinto Neto, J.V.; Campos, L.T.; Hamerschlak, N. BCL-2 as therapeutic target for hematological malignancies. J. Hematol. Oncol. 2018, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Desikan, S.P.; Daver, N.; DiNardo, C.; Kadia, T.; Konopleva, M.; Ravandi, F. Resistance to targeted therapies: Delving into FLT3 and IDH. Blood Cancer J. 2022, 12, 91. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Gerecke, C.; Fuhrmann, S.; Strifler, S.; Schmidt-Hieber, M.; Einsele, H.; Knop, S. The Diagnosis and Treatment of Multiple Myeloma. Dtsch. Arztebl. Int. 2016, 113, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Ormond Filho, A.G.; Carneiro, B.C.; Pastore, D.; Silva, I.P.; Yamashita, S.R.; Consolo, F.D.; Hungria, V.T.M.; Sandes, A.F.; Gil Rizzatti, E.; Nico, M.A.C. Whole-Body Imaging of Multiple Myeloma: Diagnostic Criteria. Radiographics 2019, 39, 1077–1097. [Google Scholar] [CrossRef]
- Zamagni, E.; Tacchetti, P.; Cavo, M. Imaging in multiple myeloma: How? When? Blood 2019, 133, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Branagan, A.; Lei, M.; Lou, U.; Raje, N. Current Treatment Strategies for Multiple Myeloma. JCO Oncol. Pract. 2020, 16, 5–14. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]
- Bruins, W.S.C.; Zweegman, S.; Mutis, T.; Van De Donk, N.W.C.J. Targeted Therapy With Immunoconjugates for Multiple Myeloma. Front. Immunol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Watson, E.; Djebbari, F.; Rampotas, A.; Ramasamy, K. BCMA-targeted therapies for multiple myeloma: Strategies to maximize efficacy and minimize adverse events. Expert Rev. Hematol. 2022, 15, 503–517. [Google Scholar] [CrossRef]
- Reynoso, E.E.; Quintero, C. How to Treat Isolated Leptomeningeal Relapse of Multiple Myeloma 11 Years after Autologous Stem Cell Transplantation (ASCT). Blood 2022, 140 (Suppl. S1), 12503. [Google Scholar] [CrossRef]
- Kornberg, M.D.; Ratchford, J.N.; Subramaniam, R.M.; Probasco, J.C. Giant cell arteritis mimicking infiltrative leptomeningeal disease of the optic nerves. BMJ Case Rep. 2015, 2015, bcr2014209160. [Google Scholar] [CrossRef]
- Fischer, S.; Weber, J.; Senn-Schönenberger, I.; Cerny, T.; Hundsberger, T. Neuroborreliosis Mimicking Leptomeningeal Carcinomatosis in a Patient With Breast Cancer: A Case Report. J. Investig. Med. High Impact Case Rep. 2014, 2, 2324709614529417. [Google Scholar] [CrossRef]
- Saltijeral, S.N.; Grosu, H.B.; De La Garza, H.; O’brien, B.; Iliescu, G. Leptomeningeal Enhancement due to Neurosarcoidosis Mimicking Malignancy. Case Rep. Med. 2020, 2020, 9513576. [Google Scholar] [CrossRef] [Green Version]
- Ungprasert, P.; Ryu, J.H.; Matteson, E.L. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 358–375. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Casals, M.; Pérez-Alvarez, R.; Kostov, B.; Gómez-De-La-Torre, R.; Feijoo-Massó, C.; Chara-Cervantes, J.; Pinilla, B.; González-García, A.; Garcia-Morillo, J.-S.; López-Dupla, M.; et al. Clinical characterization and outcomes of 85 patients with neurosarcoidosis. Sci. Rep. 2021, 11, 13735. [Google Scholar] [CrossRef]
- Bergantini, L.; Nardelli, G.; D’alessandro, M.; Montuori, G.; Piccioli, C.; Rosi, E.; Gangi, S.; Cavallaro, D.; Cameli, P.; Bargagli, E. Combined Sarcoidosis and Idiopathic Pulmonary Fibrosis (CSIPF): A New Phenotype or a Fortuitous Overlap? Scoping Review and Case Series. J. Clin. Med. 2022, 11, 2065. [Google Scholar] [CrossRef]
- Kidd, D.P. Sarcoidosis of the central nervous system: Clinical features, imaging, and CSF results. J. Neurol. 2018, 265, 1906–1915. [Google Scholar] [CrossRef]
- Galnares-Olalde, J.A.; Berebichez-Fridman, R.; Gómez-Garza, G.; Mercado, M.; Moreno-Sánchez, F.; Alegría-Loyola, M.A. Not everything is as it seems: Neurosarcoidosis presenting as leptomeningitis. Clin. Case Rep. 2018, 6, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Voortman, M.; Drent, M.; Baughman, R.P. Management of neurosarcoidosis: A clinical challenge. Curr. Opin. Neurol. 2019, 32, 475–483. [Google Scholar] [CrossRef]
- Kaplan, J.G.; DeSouza, T.G.; Farkash, A.; Shafran, B.; Pack, D.; Rehman, F.; Fuks, J.; Portenoy, R. Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J. Neuro-Oncol. 1990, 9, 225–229. [Google Scholar] [CrossRef]
- Wasserstrom, W.R.; Glass, J.P.; Posner, J.B. Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Cancer 1982, 49, 759–772. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Fomchenko, E.I.; Guerrieri, R.A.; Oliva, I.C.G. Challenges and Advances in Diagnosis and Treatment of Leptomeningeal Disease (LMD). Front. Oncol. 2022, 11, 800053. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.; Nguyen, A.; Dada, O.T.; Desai, P.D.; Ricci, J.C.; Godbole, N.B.; Pierre, K.; Lucke-Wold, B. Leptomeningeal Metastasis: A Review of the Pathophysiology, Diagnostic Methodology, and Therapeutic Landscape. Curr. Oncol. 2023, 30, 5906-5931. https://doi.org/10.3390/curroncol30060442
Nguyen A, Nguyen A, Dada OT, Desai PD, Ricci JC, Godbole NB, Pierre K, Lucke-Wold B. Leptomeningeal Metastasis: A Review of the Pathophysiology, Diagnostic Methodology, and Therapeutic Landscape. Current Oncology. 2023; 30(6):5906-5931. https://doi.org/10.3390/curroncol30060442
Chicago/Turabian StyleNguyen, Andrew, Alexander Nguyen, Oluwaferanmi T. Dada, Persis D. Desai, Jacob C. Ricci, Nikhil B. Godbole, Kevin Pierre, and Brandon Lucke-Wold. 2023. "Leptomeningeal Metastasis: A Review of the Pathophysiology, Diagnostic Methodology, and Therapeutic Landscape" Current Oncology 30, no. 6: 5906-5931. https://doi.org/10.3390/curroncol30060442
APA StyleNguyen, A., Nguyen, A., Dada, O. T., Desai, P. D., Ricci, J. C., Godbole, N. B., Pierre, K., & Lucke-Wold, B. (2023). Leptomeningeal Metastasis: A Review of the Pathophysiology, Diagnostic Methodology, and Therapeutic Landscape. Current Oncology, 30(6), 5906-5931. https://doi.org/10.3390/curroncol30060442