Evaluation of the PAH Content in Soot from Solid Fuels Combustion in Low Power Boilers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
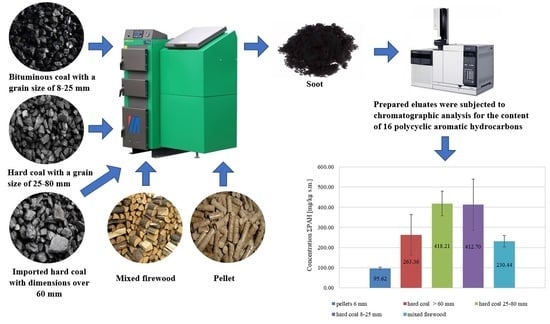
- Among the examined soot samples from the combustion of solid fuels, the lowest PAH concentration was obtained in soot samples from combustion of 6-mm pellets (95.62 ± 6.44 mg/kg DM), and the highest was obtained for 25–80 mm coal (418.21 ± 61.03 mg/kg DM), which was over 4 times higher than in the case of 6-mm pellets.
- Analysis of the compactness of the light LMW fraction showed that the smallest share of this fraction in the total concentration was recorded in samples of 6-mm pellets, representing only 5% of the total PAH total. This fraction was measured to be 12% for firewood. In the case of coals, much higher percentages of the LMW fraction were obtained.
- Higher percentages of PAH with higher molecular weights, i.e., the HMW fraction, accounted for more than 50% in each of the analyzed solid fuels.
- Considering the calculated TEQ = CEQ, MEQ and TCDD-TEQ indicators, it was found that the most toxic and carcinogenic is black carbon with the granulation of 25–80 mm, while soot samples from combustion 6-mm pellets.
- The largest ratio of ΣPAHcarc/ΣPAH was obtained in the case of soot samples from the combustion of 6-mm pellets, while the smallest was from the combustion of coal with a granulation of 8–25 mm.
Author Contributions
Funding
Conflicts of Interest
References
- Skotak, K.; Degórska, A.; Ulańczyk, R.; Pecka, T. Soot as an indicator of human activity for life and environment. Chem. Ind. 2016, 95, 548–553. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Kuznetsov, G.V.; Strizhak, P.A. Ignition Characteristics of Coal—Water Slurry Containing Petrochemicals Based on Coal of Varying Degrees of Metamorphism. Energy Fuels 2016, 30, 6808. [Google Scholar] [CrossRef]
- Dzikuć, M.; Kułyk, P.; Dzikuć, M.; Urban, S.; Piwowar, A. Associated with Low Emission Reductions in Poland’s Lubuskie Voivodeship. Pol. J. Environ. Stud. 2019, 28, 65–72. [Google Scholar] [CrossRef]
- Schulz, F.; Commodo, M.; Kaiser, K.; De Falco, G.; Minutolo, P.; Meyer, G.; Gross, L.; Anna, A.D. Insights into incipient soot formation by atomic force microscopy. Proc. Combust. Inst. 2019, 37, 885–892. [Google Scholar] [CrossRef]
- Commodo, M.; Kaiser, K.; De Falco, G.; Minutolo, P.; Schulz, F.; Leo Gross, A.D.A. On the early stages of soot formation: Molecular structure elucidation by high-resolution atomic force microscopy. Combust. Flame 2019, 205, 154–164. [Google Scholar] [CrossRef]
- Michelsen, H.A. Probing soot formation, chemical and physical evolution, and oxidation: A review of in situ diagnostic techniques and needs. Proc. Combust. Inst. 2017, 36, 717–735. [Google Scholar] [CrossRef] [Green Version]
- European Environment. The European Environment State and Outlook 2015 Synthesis Report; Environmental Protection Agency (EPA): Copenhagen, Denmark, 2015.
- Zhan, C.; Zhang, J.; Zheng, J. Characterization of carbonaceous fractions in PM2.5 and PM10 over a typical industrial city in central China. Environ. Sci. Pollut. Res. 2019, 26, 16855. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Brooks, P. Characterization of carbonaceous combustion residues: II. Nonpolar organic compounds. Chemosphere 2003, 53, 447–448. [Google Scholar] [CrossRef]
- Lokeshappa, B.; Dikshit, A.K. Fate of metals in coal fly ash ponds. Int. Conf. Environ. Sci. Dev. 2012, 3, 43–48. [Google Scholar]
- Status of Black Carbon Monitoring in Ambient Air in Europe; Technical Report No 18/2013; European Environment Agency (EEA): Copenhagen, Denmark, 2013.
- Maricq, M.M.; Xu, N. The effective density and fractal dimension of soot particles from premixed flames and motor vehicle exhaust. J. Aerosol Sci. 2004, 35, 1251–1274. [Google Scholar] [CrossRef]
- Wainwright, M.; Alharbi, S.; Wickramasinghe, N.C. How do microorganisms reach the stratosphere? Int. J. Astrobiol. 2006, 5, 1–3. [Google Scholar] [CrossRef]
- Lewtas, J. Air pollution combustion emissions: Characterization of causativeagents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res. 2007, 636, 95–133. [Google Scholar] [CrossRef]
- Tiwari, S.; Pipal, A.S.; Srivastava, A.K.; Bisht, D.S.; Pandithurai, G. Determination of wood burning and fossil fuel contribution of black carbon at Delhi, India using aerosol light absorption technique. Environ. Sci. Pollut. Res. 2015, 22, 2846. [Google Scholar] [CrossRef]
- Pope, C.A.; Ezzati, M.; Dockery, D. Fine-Particulate Air Pollution and Life Expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Pratt, M.M.; John, K.; MacLean, A.B.; Afework, S.; Phillips, D.H.; Poirier, M.C. Polycyclic aromatic hydrocarbon (PAH) Exposure and DNA adduct semi-quantitation in archived human tissues. Int. J. Environ. Res. Public Health 2011, 8, 2675–2691. [Google Scholar] [CrossRef]
- Anenberg, S. Technology: Clean stoves benefit climate and health. Nature 2012, 490, 343. [Google Scholar] [CrossRef]
- Schmidt, C.W. Black carbon: The dark horse of climate change drivers. Environ. Health Perspect. 2011, 119, A172–A175. [Google Scholar] [CrossRef]
- Kubica, R.; Kubica, K.; Kacprzyk, W. Limitation of black carbon emissions from solid fuel combustion in small plants. Chem. Ind. 2016, 95, 472–479. [Google Scholar] [CrossRef]
- Poland’s National Inventory Report 2019 Greenhouse Gas Inventory for 1988–2017. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwiNsaLm1tnlAhWp-GEKHbupDqwQFjAAegQIAhAC&url=https%3A%2F%2Fwww.kobize.pl%2Fuploads%2Fmaterialy%2Fmaterialy_do_pobrania%2Fkrajowa_inwentaryzacja_emisji%2FNIR_POL_2019_23.05.2019.pdf&usg=AOvVaw0uTJTEVB488hep1GpokGdt (accessed on 6 October 2019).
- Dołżyńska, M.; Obidziński, S.; Kowczyk-Sadowy, M.; Krasowska, M. Densification and Combustion of Cherry Stones. Energies 2019, 12, 3042. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Kozielska, B.; Klejnowski, K. Concentration, origin and health hazard from fine particle-bound PAH at three characteristic sites in Southern Poland. Bull. Environ. Contam. Toxicol. 2013, 91, 349–355. [Google Scholar] [CrossRef]
- Durant, J.L.; Busby, W.F., Jr.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. Genet. Toxicol. 1996, 371, 123–157. [Google Scholar] [CrossRef]
- Willett, K.L.; Gardinali, P.R.; Sericano, J.L.; Wade, T.L.; Safe, S.H. Characterization of the H4IIE rat hepatoma cell bioassay for evaluation of environmental samples containing polynuclear aromatic hydrocarbons (PAHs). Arch. Environ. Contam. Toxicol. 1996, 32, 442–448. [Google Scholar] [CrossRef]
- Bourotte, C.; Forti, M.C.; Taniguchi, S.; Bícego, M.C.; Lotufo, P.A. A wintertime study of PAHs in fine and coarse aerosols in São Paulo city, Brazil. Atmos. Environ. 2005, 39, 3799–3811. [Google Scholar] [CrossRef]
- Biache, C.; Mansuy-Huault, L.; Faure, P. Impact of oxidation and biodegradation on the most commonly used polycyclic aromatic hydrocarbons (PAH) diagnostic ratios: Implications for the source identification. J. Hazard. Mater. 2014, 267, 31–39. [Google Scholar] [CrossRef]
- Kubiak, S.M. Polycyclic Aromatic Hydrocarbons (PAHs)—their occurrence in the environment and food. Probl. Hig. Epidemiol. 2013, 94, 31–36. [Google Scholar]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Jyethi, D.S.; Khillare, P.S.; Sarkar, S. Risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons in school children. Environ. Sci. Pollut. Res. 2014, 21, 366–378. [Google Scholar] [CrossRef]
- Wójcik, W.; Kotyra, A.; Smolarz, A.; Gromaszek, K. Modern methods of monitoring and control of the solid fuels combustion process in order to reduce its impact on the natural environment. Annu. Set Environ. Prot. 2011, 13, 1559–1576. [Google Scholar]
- Kakareka, S.V.; Kukharchyk, T.I.; Khomich, V.S. Study of PAH emission from the solid fuels combustion in residential furnaces. Environ. Pollut. 2005, 133, 383–387. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Baran, S. Degradation of individual polycyclic aromatic hydrocarbons (PAHs) in soil polluted with aircraft fuel. Pol. J. Environ. Stud. 2003, 12, 431–437. [Google Scholar]
- Lamichhane, S.; Krishna, K.C.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef]
- Kamińska, G.; Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Removal and fate of PAHs during mechanical-biological wastewater treatment. Proc. ECOpole 2016, 10, 653–660. [Google Scholar] [CrossRef]
- Kozielska, B.; Rogula-Kozłowska, W.; Rogula-Kopiec, P.; Jureczko, I. Polycyclic Aromatic Hydrocarbons in different airborne particulate matter fractions of areas dominated by communication emissions. Ecol. Eng. 2016, 49, 25–32. [Google Scholar] [CrossRef]
- Brzeźnicki, S.; Bonczarowska, M.; Gromiec, J.P. Maximum levels for Polycyclic Aromatic Hydrocarbons (PAHs). Current legal situation and proposed amendments. Med. Pr. 2009, 60, 179–185. [Google Scholar]




| Working Conditions | Value |
| carrier gas | helium with purity 6.0 |
| carrier gas flow rate through the column | 1 mL/min (splitless) |
| volume of the injected sample | 1 μL |
| dispenser temperature | 260 °C |
| ion source temperature | 230 °C |
| transfer line temperature | 300 °C |
| quadrupole temperature | 150 °C |
| scanning mode | single ion monitoring (SIM—selected ion monitoring) |
| The temperature program was set as follows | |
| initial temperature of the column furnace | 60 °C, 2 min isothermal |
| temperature increase | 30 °C/min to 120 °C, from 120 °C temperature increase 5 °C/min to 300 °C |
| end temperature of the column furnace | 300 °C, isothermal 15 min |
| Ordinal Number | Polycyclic Aromatic Hydrocarbons | Short Name | Structure (Number of Rings) | Molecular Weight (g/mole) | Solubility (mg/L) | Vapor Pressure (mm Hg) |
|---|---|---|---|---|---|---|
| 1 | Naphthalene | Nap | 2 | 128.17 | 31 | 8.89 × 10−2 |
| 2 | Acenaphthylene | Acy | 3 | 152.20 | 16.1 | 2.90 × 10−2 |
| 3 | Acenaphthene | Ace | 3 | 154.21 | 3.8 | 3.75 × 10−3 |
| 4 | Fluorene | Flu | 3 | 166.22 | 1.9 | 3.24 × 10−3 |
| 5 | Phenanthrene | Phe | 3 | 178.23 | 1.1 | 6.80 × 10−4 |
| 6 | Anthracene | Ant | 3 | 178.23 | 0.045 | 2.55 × 10−5 |
| 7 | Fluoranthene | Flr | 4 | 202.26 | 0.26 | 8.13 × 10−6 |
| 8 | Pyrene | Pyr | 4 | 202.26 | 0.132 | 4.25 × 10−6 |
| 9 | Benzo(a)anthracene | B(a)A | 4 | 228.29 | 0.011 | 1.54 × 10−7 |
| 10 | Chrysene | Chr | 4 | 228.29 | 0.0015 | 7.80 × 10−9 |
| 11 | Benzo(b)fluoranthene | B(b)F | 5 | 252.32 | 0.0015 | 8.06 × 10−8 |
| 12 | Benzo(k)fluoranthene | B(k)F | 5 | 252.32 | 0.0008 | 9.59 × 10−11 |
| 13 | Benzo(a)pyrene | B(a)P | 5 | 252.32 | 0.0038 | 4.89 × 10−9 |
| 14 | Indeno[1,2,3-cd]pyrene | I(cd)P | 6 | 276.34 | 0.062 | 1.40 × 10−10 |
| 15 | Dibenz(a,h)anthracene | D(ah)A | 6 | 278.35 | 0.0005 | 2.10 × 10−11 |
| 16 | Benzo(g,h,i)perylene | B(ghi)P | 6 | 276.34 | 0.00026 | 1.00 × 10−10 |
| mg/kg DM | Pellet 6 mm | Hard Coal > 60 mm | Hard Coal 25–80 mm | Hard Coal 8–25 mm | Mixed Firewood |
|---|---|---|---|---|---|
| Naphthalene | 0.50 ± 0.20 | 2.52 ± 3.04 | 1.20 ± 0.04 | 2.17 ± 0.46 | 1.33 ± 0.08 |
| Acenaphthylene | 0.63 ± 0.07 | 10.77 ± 4.46 | 4.48 ± 0.45 | 13.01 ± 5.83 | 3.05 ± 0.33 |
| Acenaphthene | 0.05 ± 0.002 | 1.28 ± 1.62 | 0.29 ± 0.03 | 0.59 ± 0.26 | 0.15 ± 0.02 |
| Fluorene | 0.07 ± 0.002 | 9.62 ± 13.34 | 0.86 ± 0.08 | 7.71 ± 9.23 | 0.27 ± 0.02 |
| Phenanthrene | 2.87 ± 0.12 | 50.05 ± 12.44 | 47.5 ± 5.69 | 52.49 ± 11.67 | 18.82 ± 2.45 |
| Anthracene | 0.78 ± 0.05 | 13.56 ± 5.95 | 17.49 ± 2.15 | 19.28 ± 6.44 | 4.39 ± 0.57 |
| Fluoranthene | 5.06 ± 0.30 | 17.95 ± 5.03 | 46.34 ± 6.25 | 36.38 ± 9.06 | 14.18 ± 2.23 |
| Pyrene | 5.23 ± 0.61 | 16.42 ± 2.51 | 41.12 ± 5.39 | 32.01 ± 9.68 | 11.84 ± 1.90 |
| Benzo(a)anthracene | 3.70 ± 0.09 | 13.05 ± 9.14 | 26.17 ± 3.86 | 18.84 ± 5.65 | 8.44 ± 1.27 |
| Chrysene | 4.52 ± 0.11 | 12.04 ± 7.09 | 24.07 ± 3.61 | 17.09 ± 5.07 | 8.73 ± 1.24 |
| Benzo(b)fluoranthene | 13.07 ± 3.97 | 22.40 ± 7.40 | 33.09 ± 5.78 | 29.04 ± 8.70 | 32.87 ± 5.29 |
| Benzo(k)fluoranthene | 15.58 ± 0.55 | 27.02 ± 12.85 | 49.58 ± 7.77 | 44.49 ± 12.95 | 28.03 ± 4.50 |
| Benzo(a)pyrene | 12.67 ± 0.06 | 30.87 ± 6.71 | 60.48 ± 10.05 | 58.55 ± 17.84 | 36.53 ± 5.72 |
| Indeno[1,2,3-cd]pyrene | 16.54 ± 2.69 | 20.42 ± 6.35 | 36.70 ± 6.02 | 44.82 ± 9.48 | 33.94 ± 2.56 |
| Dibenz(a,h)anthracene | 1.32 ± 0.15 | 2.45 ± 0.97 | 6.69 ± 0.99 | 6.06 ± 2.42 | 4.39 ± 0.33 |
| Benzo(g,h,i)perylene | 13.01 ± 1.95 | 12.93 ± 2.63 | 22.12 ± 3.19 | 30.16 ± 5.60 | 23.48 ± 1.50 |
| Σ16PAH | 95.62 ± 6.44 | 263.36 ± 91.00 | 418.21 ± 61.03 | 412.70 ± 127.16 | 230.44 ± 28.62 |
| ΣLMW | 4.90 ± 0.42 | 87.81 ± 24.89 | 71.86 ± 6.24 | 95.26 ± 32.92 | 28.01 ± 2.81 |
| ΣHMW | 90.72 ± 5.99 | 175.55 ± 60.16 | 346.35 ± 52.67 | 317.43 ± 58.32 | 188.26 ± 25.31 |
| Indicator | Pellet 6 mm | Hard Coal > 60 mm | Hard Coal 25–80 mm | Hard Coal 8–25 mm | Mixed Firewood |
|---|---|---|---|---|---|
| TEQ = CEQ | 28.39 ± 1.19 | 103.06 ± 37.95 | 158.76 ± 23.34 | 158.53 ± 31.77 | 90.16 ± 11.12 |
| MEQ | 26.02 ± 1.68 | 50.22 ± 13.47 | 94.28 ± 15.47 | 93.93 ± 16.65 | 64.93 ± 8.56 |
| TCDD-TEQ | 0.19 ± 0.01 | 0.33 ± 0.06 | 0.61 ± 0.09 | 0.55 ± 0.11 | 0.39 ± 0.06 |
| ΣPAHcarc/ΣPAH | 0.70 ± 0.02 | 0.60 ± 0.15 | 0.56 ± 0.01 | 0.53 ± 0.01 | 0.66 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatyłowicz, E.; Skoczko, I. Evaluation of the PAH Content in Soot from Solid Fuels Combustion in Low Power Boilers. Energies 2019, 12, 4254. https://doi.org/10.3390/en12224254
Szatyłowicz E, Skoczko I. Evaluation of the PAH Content in Soot from Solid Fuels Combustion in Low Power Boilers. Energies. 2019; 12(22):4254. https://doi.org/10.3390/en12224254
Chicago/Turabian StyleSzatyłowicz, Ewa, and Iwona Skoczko. 2019. "Evaluation of the PAH Content in Soot from Solid Fuels Combustion in Low Power Boilers" Energies 12, no. 22: 4254. https://doi.org/10.3390/en12224254
APA StyleSzatyłowicz, E., & Skoczko, I. (2019). Evaluation of the PAH Content in Soot from Solid Fuels Combustion in Low Power Boilers. Energies, 12(22), 4254. https://doi.org/10.3390/en12224254