Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production
Abstract
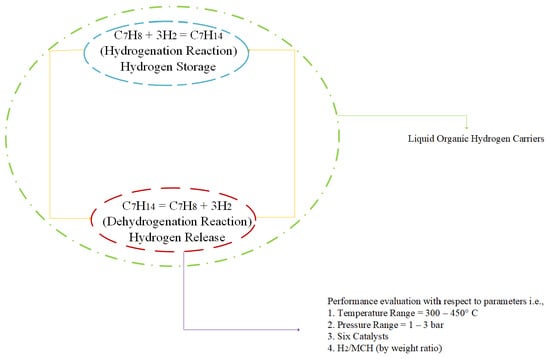
:1. Introduction
- MCH and toluene exist in liquid form, and have no handling issues, unlike the naphthalene–decalin system, in which naphthalene exists in a solid state, thus causing handling issues, making this system unfavorable for operation.
- Comparatively, benzene is more toxic than methylcyclohexane. As reported by US health exposure limits (NIOSH), the permissible exposure limit of benzene is 1 ppm and methylcyclohexane is 500 ppm. An exposure limit of 500 ppm of benzene causes immediate danger, while 1200 ppm of MCH does the same.
2. Simulation Study
- Pre-heater: heat is exchanged between methylcyclohexane (MCH-1) with saturated steam (Steam) from the exhaust of the extraction type steam turbine.
- Vaporizer: the methylcyclohexane (MCH-2) exchanges heat with steam (Steam-1) in the vaporizer.
- Superheater: the methylcyclohexane (MCH-3) exchanges heat with products of dehydrogenation reactor (DeH-1) to superheat the methylcyclohexane for the final pumping into the dehydrogenation reactor.
- Pressure range: 1–3 bars.
- Temperature range: 300–450 °C.
- Concentration of hydrogen in the feed: H2/MCH ratio (by weight) at a value of 0 and 0.5.
3. Results
- H2/MCH ratio = 0
- H2/MCH ratio = 0.5
- Temperature range = 360–390 °C
- Pressure range = 1.8–2 bar
- Addition/concentration of hydrogen, i.e., H2/MCH = 0.5 (by weight)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| T | Temperature (°C) |
| P | Pressure (bar) |
| LOHC | Liquid organic hydrogen carriers |
| MTH | Methylcyclohexane–toluene–hydrogen |
| MCH | Methylcyclohexane |
| DBT | Dibenzyl toluene |
| PDBT | Perhydro–dibenzyl toluene |
| BET | Brunauer–Emmett–Teller |
| −r | Rate of dehydrogenation reaction (mol kg−1 s−1) |
| k | Rate constant for the MCH dehydrogenation reaction |
| K | Equilibrium constant of MCH dehydrogenation reaction, bar3 |
| K’ | Lumped equilibrium constant, bar−3 |
| td | Online reaction deactivation time, s |
| kWh x/kWh hyd | Calculation of the energy losses (Ex) occurring during a process step (kWhx) relative to the amount of transported energy (kWh hyd) (expressed by the lower heating value of the hydrogen, LHV, 33 kWh/kg) |
| PL | Power Law |
| LHHW | Langmuir–Hinshelwood–Hougen–Watson |
References
- Müller, K.; Arlt, W. Status and Development in Hydrogen Transport and Storage for Energy Applications. Energy Technol. 2013, 1, 501–511. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrog. Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Thiele, S.; Wasserscheid, P. Evaluations of Concepts for the Integration of Fuel Cells in Liquid Organic Hydrogen Carrier Systems. Energy Fuels 2019, 33, 10324–10330. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrog. Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Preuster, P.; Fang, Q.; Peters, R.; Deja, R.; Nguyen, V.N.; Blum, L.; Stolten, D.; Wasserscheid, P. Solid oxide fuel cell operating on liquid organic hydrogen carrier-based hydrogen—making full use of heat integration potentials. Int. J. Hydrog. Energy 2018, 43, 1758–1768. [Google Scholar] [CrossRef]
- Michalska, K.; Kowalik, P.; Konkol, M.; Próchniak, W.; Stołecki, K.; Słowik, G.; Borowiecki, T. The effect of copper on benzene hydrogenation to cyclohexane over Ni/Al2O3 catalyst. Appl. Catal. A Gen. 2016, 523, 54–60. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Hu, P.; Fogler, E.; Diskin-Posner, Y.; Iron, M.A.; Milstein, D. A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation. Nat. Commun. 2015, 6, 6859. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.R.; Cresswell, D.L. Options for on-board use of hydrogen based on the methylcyclohexane-totoluene-hydrogen system. Int. J. Green Energy 2013, 10, 177–189. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. An overview of the kinetics and catalysis of hydrogen storage on organic liquids. Can. J. Chem. Eng. 2013, 91, 1477–1490. [Google Scholar] [CrossRef]
- Taube, M.; Rippin, D.W.T.; Cresswell, D.L.; Knecht, W. A system of hydrogen-powered vehicles with liquid organic hydrides. Int. J. Hydrog. Energy 1983, 8, 213–225. [Google Scholar] [CrossRef]
- Jothimurugesan, K.; Bhatia, S.; Srivastava, R.D. Kinetics of dehydrogenation of methylcyclohexane over a platinum-rhenium-alumina catalyst in the presence of added hydrogen. Ind. Eng. Chem. Fundam. 1985, 24, 433–438. [Google Scholar] [CrossRef]
- Mizsey, P.; Cuellar, A.; Newson, E.; Hottinger, P.; Truong, T.B.; von Roth, F. Fixed bed reactor modelling and experimental data for catalytic dehydrogenation in seasonal energy storage applications. Comput. Chem. Eng. 1999, 23, S379–S382. [Google Scholar] [CrossRef]
- Usman, M.R. Methylcyclohexane dehydrogenation over commercial 0.3 Wt% Pt/Al2O3 catalyst. Proc. Pak. Acad. Sci. 2011, 48, 13–17. [Google Scholar]
- Usman, M.R.; Aslam, R.; Alotaibi, F. Hydrogen Storage in a Recyclable Organic Hydride: Kinetic Modeling of Methylcyclohexane Dehydrogenation Over 1.0 wt% Pt/θ-Al2O3. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 2264–2271. [Google Scholar] [CrossRef]
- Usman, M.R. The Catalytic Dehydrogenation of Methylcyclohexane over Monometallic Catalysts for On-board Hydrogen Storage, Production, and Utilization. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 2231–2238. [Google Scholar] [CrossRef]
- Usman, M.R.; Alotaibi, F.M.; Aslam, R. Dehydrogenation-hydrogenation of methylcyclohexane-toluene system on 1.0 wt% Pt/zeolite beta catalyst. Prog. React. Kinet. Mech. 2015, 40, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.R.; Cresswell, D.L. Prototype reactor simulation for on-board use of hydrogen in a hybrid MTH (methylcyclohexane–toluene–hydrogen)–gasoline system and a simplified dynamic modeling for the startup. Chem. Eng. Res. Des. 2015, 104, 125–138. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Tsuru, T.; Kang, D.-Y. Simulation and design of catalytic membrane reactor for hydrogen production via methylcyclohexane dehydrogenation. Int. J. Hydrog. Energy 2017, 42, 26296–26307. [Google Scholar] [CrossRef]
- Wang, W.; Miao, L.; Wu, K.; Chen, G.; Huang, Y.; Yang, Y. Hydrogen evolution in the dehydrogenation of methylcyclohexane over Pt/CeMgAlO catalysts derived from their layered double hydroxides. Int. J. Hydrog. Energy 2019, 44, 2918–2925. [Google Scholar] [CrossRef]
- Obara, S. Energy and exergy flows of a hydrogen supply chain with truck transportation of ammonia or methyl cyclohexane. Energy 2019, 174, 848–860. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Prananto, L.A.; Mufrodi, Z.; Budiman, A.; Oda, T.; Aziz, M. Highly energy-efficient combination of dehydrogenation of methylcyclohexane and hydrogen-based power generation. Appl. Energy 2018, 226, 31–38. [Google Scholar] [CrossRef]
- Prananto, L.A.; Biddinika, M.K.; Aziz, M. Combined dehydrogenation and hydrogen-based power generation. Energy Procedia 2017, 142, 1603–1608. [Google Scholar] [CrossRef]
- Vajda, S.; Pellin, M.J.; Greeley, J.P.; Marshall, C.L.; Curtiss, L.A.; Ballentine, G.A.; Elam, J.W.; Mucherie, S.C.; Redfern, P.C.; Mehmood, F.; et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213–216. [Google Scholar] [CrossRef]
- Kuo CTe Lu, Y.; Kovarik, L.; Engelhard, M.; Karim, A.M. Structure Sensitivity of Acetylene Semi-Hydrogenation on Pt Single Atoms and Subnanometer Clusters. ACS Catal. 2019, 9, 11030–11041. [Google Scholar]
- Aquilanti, V.; Coutinho, N.D.; Carvalho-Silva, V.H. Kinetics of low-temperature transitions and a reaction rate theory from non-equilibrium distributions. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375. [Google Scholar] [CrossRef]
- Chen, B.; Hoffmann, R.; Cammi, R. The Effect of Pressure on Organic Reactions in Fluids—A New Theoretical Perspective. Angew. Chem. Int. Ed. 2017, 56, 11126–11142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, A.F.; Özkasikci, D.; Fürtauer, S.; Reinelt, M. The Effect of Deprotonation on the Reaction Kinetics of an Oxygen Scavenger Based on Gallic Acid. Front. Chem. 2019, 7, 680. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.H.; Hussain, M.; Maafa, I.M.; Aslam, R. Integration of hydrogenation and dehydrogenation system for hydrogen storage and electricity generation-simulation study. Int. J. Hydrog. Energy 2019, 44, 20213–20222. [Google Scholar] [CrossRef]
- Usman, M.R.; Cresswell, D.L.; Garforth, A.A. By-Products Formation in the Dehydrogenation of Methylcyclohexane. Pet. Sci. Technol. 2011, 29, 2247–2257. [Google Scholar] [CrossRef]








| Fuel | Environmental Impact (ienv) |
|---|---|
| Hydrogen | 0.95 (no water) 0.45 |
| Coal | 0.82 (no water) 0.78 |
| Gasoline | 0.76 (no water) 0.72 |
| Methane | 0.90 (no water) 0.80 |
| Description | Unit | Liquid Organic Hydrogen Carriers | Compressed Hydrogen Storage | Liquid Hydrogen Storage |
|---|---|---|---|---|
| Energy Demand | kWh x/kWh hyd | 1.1% | 3.5% | 21% |
| Cost Estimation | €/kg hydrogen a | 0.238 | 0.243 | 0.732 |
| Characteristic | LOHC | Liquid H2 | Ammonia (NH3) |
|---|---|---|---|
| Purpose |
|
|
|
| Infrastructure |
|
|
|
| Auto-Ignition Temperature |
|
|
|
| Advantages |
|
|
|
| Challenges |
|
|
|
| Development Stage |
|
|
|
| Prospects |
|
|
|
| Characteristics | Benzene–Cyclohexane System | Naphthalene–Decalin System | DBT–PDBT System | MCH–Toluene System |
|---|---|---|---|---|
| Phase under ambient conditions | Liquid | Solid + Liquid | Liquid | Liquid |
| Temperature (°C) | 150–250 | 150–250 | 180 | 200–300 |
| Pressure (bar) | 10–50 | 20–50 | 10–50 | 10–50 |
| Volumetric H2 density (kg-H2.m−3) | 55.9 | 65.4 | 57 | 47.4 |
| Gravimetric H2 density (wt. %) | 7.20 | 7.29 | 6.2 | 6.16 |
| Heat of reaction (kJ.mol−1) | 205.9 | 319.5 | 588.5 | 204.8 |
| Challenges | High melting point and toxicity | Dehydrogenation process requires high energy consumption, solid in nature, difficult handling. | Dehydrogenation process requires high energy consumption. | Volatile and inflammable. |
| Catalyst | MCH Conversion (XA) (%) | Byproduct Selectivity (%) |
|---|---|---|
| 1 wt.% Pt/γ-Al2O3 | 92 | 0.63 |
| 1 wt.% Pt/θ-Al2O3 | 91 | 0.10 |
| 1 wt.% Pt/β-zeolite | 73 | 22.48 |
| 20 wt.% Ni/γ-Al2O3 | 31 | 7.87 |
| Sr. No. | Catalyst Type | Model | Kinetic Parameters | Reference |
|---|---|---|---|---|
| Catalyst-1 | 0.3 wt. % commercial Pt/Al2O3 | Power Law | k = 1.65 × 10−5 Ea = 100.6 | [18] |
| Catalyst-2 | Commercial sulfided/spherical alumina support | Power Law | k = 2.335 × 10−6 Ea = 200 | [17] |
| Catalyst-3 | 1 wt. % Pt/θ-Al2O3 | LHHW | k = 6.60 × 10−5 Ea = 50.2 | [19] |
| Catalyst-4 | 1 wt. % Pt/β-Zeolite | Power Law LHHW | k = 1.143 × 10−5 Ea = 6.0 K’ = 0.3088 | [21] |
| Catalyst-5 | 1 wt. % Pt/γ-Al2O3 | LHHW | k = 4.064 × 10−5 Ea = 54.55 K’ = 0.32 | [22] |
| Catalyst-6 | 0.3 wt. % Pt-Re/Al2O3 | Power Law | k = 1.336 × 10−5 Ea = 51.9 | [16] |
| Catalyst Type | P = 1 bar | P = 1.8 bar | P = 3 bar |
|---|---|---|---|
| % MCH Conversion (No Hydrogen in Feed) | |||
| Catalyst-1 | 3 | 3 | 3 |
| Catalyst-2 | 5 | 5 | 6 |
| Catalyst-3 | 1 | 1 | 1 |
| Catalyst-4 | 4 | 4 | 4 |
| Catalyst-5 | 2 | 2 | 2 |
| Catalyst-6 | 6 | 6 | 5 |
| % MCH Conversion (With Hydrogen in Feed) | |||
| Catalyst-1 | 3 | 3 | 4 |
| Catalyst-2 | 5 | 5 | 6 |
| Catalyst-3 | 1 | 1 | 1 |
| Catalyst-4 | 4 | 4 | 3 |
| Catalyst-5 | 2 | 2 | 2 |
| Catalyst-6 | 6 | 6 | 5 |
| Catalyst Number | Catalyst Type | Rankings (No H2 in the Feed) | Ranking (with H2 in the Feed) |
|---|---|---|---|
| Catalyst-1 | 0.3 wt. % Pt/γ-Al2O3 | 3 | 3 |
| Catalyst-2 | Sulfided Pt/Al2O3 | 5 | 5 |
| Catalyst-3 | 1 wt. % Pt/θ-Al2O3 | 1 | 1 |
| Catalyst-4 | 1 wt. % Pt/β-Zeolite | 4 | 4 |
| Catalyst-5 | 1 wt. % Pt/γ-Al2O3 | 2 | 2 |
| Catalyst-6 | 0.3 wt. % Pt + Re/Al2O3 | 6 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamayun, M.H.; Maafa, I.M.; Hussain, M.; Aslam, R. Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production. Energies 2020, 13, 206. https://doi.org/10.3390/en13010206
Hamayun MH, Maafa IM, Hussain M, Aslam R. Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production. Energies. 2020; 13(1):206. https://doi.org/10.3390/en13010206
Chicago/Turabian StyleHamayun, Muhammad Haris, Ibrahim M. Maafa, Murid Hussain, and Rabya Aslam. 2020. "Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production" Energies 13, no. 1: 206. https://doi.org/10.3390/en13010206
APA StyleHamayun, M. H., Maafa, I. M., Hussain, M., & Aslam, R. (2020). Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production. Energies, 13(1), 206. https://doi.org/10.3390/en13010206