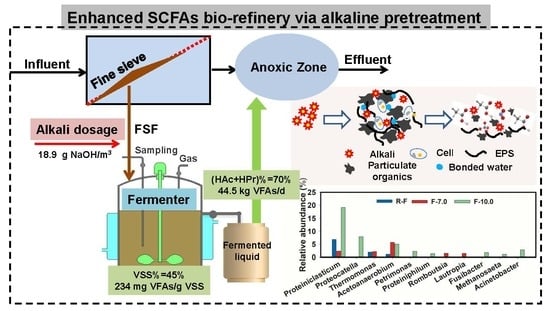
Intensification of Short Chain Fatty Acid Production during the Alkaline Pretreatment of Fine-Sieving Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sludge Collection
2.2. Batch Fermentation of Alkaline Pretreated FSF
2.3. Analysis of Microbial Community Structure
2.4. Mass Balance and Potential Benefits Evaluation
2.5. Analytical Methods and Statistical Analysis
3. Results and Discussion
3.1. Enhanced SCFAs Production and Composition under Alkaline Condition
3.2. Acceleration of FSF Hydrolysis and Inhibition of Methane Generation
3.3. Microbial Community Structure
3.4. Significance and Potential Benefits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviation | Name |
| BNR | Biological nutrient removal |
| WWTPs | Wastewater treatment plants |
| ECS | External carbon sources |
| SCFAs | Short chain fatty acids |
| COD | Chemical oxygen demand |
| WAS | Waste activated sludge |
| AD | Anaerobic digestion |
| FSF | Fine-sieving fractions |
| TCOD | Total COD |
| PCOD | Particulate COD |
| TSS | Total suspended solids |
| SBR | Sequencing batch reactor |
| SCOD | Soluble COD |
| VSS | Volatile suspended solids |
| ISS | Inorganic suspended solids |
| PCR | Polymerase chain reaction |
References
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitrification, denitrification and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef]
- Hu, X.; Wisniewski, K.; Czerwionka, K.; Zhou, Q.; Xie, L.; Makinia, J. Modeling the Effect of External Carbon Source Addition under Different Electron Acceptor Conditions in Biological Nutrient Removal Activated Sludge Systems. Environ. Sci. Technol. 2016, 50, 1887–1896. [Google Scholar] [CrossRef]
- Flores-Alsina, X.; Corominas, L.; Snip, L.; Vanrolleghem, P.A. Including greenhouse gas emissions during benchmarking of wastewater treatment plant control strategies. Water Res. 2011, 45, 4700–4710. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Y.; Sun, M.; Gao, M.; Zhao, Y.; She, Z. Enhancing denitrification with waste sludge carbon source: The substrate metabolism process and mechanisms. Environ. Sci. Pollut. Res. 2018, 25, 13079–13092. [Google Scholar] [CrossRef] [PubMed]
- Zubrowska-Sudol, M.; Walczak, J. Enhancing combined biological nitrogen and phosphorus removal from wastewater by applying mechanically disintegrated excess sludge. Water Res. 2015, 76, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD capture: A feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Robinson, Z.P.; Wang, X.; Wu, J.; Li, F. The feasibility and challenges of energy self-sufficient wastewater treatment plants. Appl. Energy 2017, 204, 1463–1475. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Genon, G.; Lorenzi, E.; Novarino, D.; Scibilia, G.; Zanetti, M. Improvement of anaerobic digestion of sewage sludge in a wastewater treatment plant by means of mechanical and thermal pre-treatments: Performance, energy and economical assessment. Bioresour. Technol. 2015, 175, 298–308. [Google Scholar] [CrossRef]
- Yi, L.; Luo, X.; Huang, X.; Wang, D.; Zhang, W. Life Cycle Assessment of a municipal wastewater treatment plant: A case study in Suzhou, China. J. Clean. Prod. 2013, 57, 221–227. [Google Scholar]
- Lin, L.; Li, R.H.; Li, X.Y. Recovery of organic resources from sewage sludge of Al-enhanced primary sedimentation by alkali pretreatment and acidogenic fermentation. J. Clean. Prod. 2018, 172, 3334–3341. [Google Scholar] [CrossRef]
- Ruiken, C.J.; Breuer, G.; Klaversma, E.; Santiago, T.; Loosdrecht, M.C.M.V. Sieving wastewater—Cellulose recovery, economic and energy evaluation. Water Res. 2013, 47, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Crutchik, D.; Frison, N.; Eusebi, A.L.; Fatone, F. Biorefinery of cellulosic primary sludge towards targeted Short Chain Fatty Acids, phosphorus and methane recovery. Water Res. 2018, 136, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Remy, C.; Boulestreau, M.; Lesjean, B. Proof of concept for a new energy-positive wastewater treatment scheme. Water Sci. Technol. 2014, 70, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, B.; Jiao, D.; Sun, G.; Wang, B.; Wang, X.C. Characterization of microflora and transformation of organic matters in urban sewer system. Water Res. 2015, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Jin, P.K.; Ngo, H.H.; Shi, X.; Guo, W.S.; Yang, S.J.; Wang, X.C.; Wang, X.; Dzakpasu, M.; Yang, W.N.; et al. Transformation and utilization of slowly biodegradable organic matters in biological sewage treatment of anaerobic anoxic oxic systems. Bioresour. Technol. 2016, 218, 53–61. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ji, F.-y.; He, X.-l.; Zhou, W.-W.; Xu, X.; Lai, M.-S. Validation of accumulation models for inorganic suspended solids of different particle size in an activated sludge system. Bioresour. Technol. 2013, 149, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rusten, B.; Razafimanantsoa, V.A.; Andriamiarinjaka, M.A.; Otis, C.L.; Sahu, A.K.; Bilstad, T. Impact of fine mesh sieve primary treatment on nitrogen removal in moving bed biofilm reactors. Water Sci. Technol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Jie, W.; Peng, Y.; Ren, N.; Li, B. Volatile fatty acids (VFAs) accumulation and microbial community structure of excess sludge (ES) at different pHs. Bioresour. Technol. 2014, 152, 124–129. [Google Scholar] [CrossRef]
- Wu, H.; Gao, J.; Yang, D.; Zhou, Q.; Liu, W. Alkaline fermentation of primary sludge for short-chain fatty acids accumulation and mechanism. Chem. Eng. J. 2010, 160, 1–7. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, Y.; Zhang, H.; Jiang, S.; Zhou, Q.; Gu, G. Improved bioproduction of short-chain fatty acids (SCFAs) from excess sludge under alkaline conditions. Environ. Sci. Technol. 2006, 40, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, A.; Liu, H.; Wang, S.; Liu, W.; Wang, A.; Yue, X. Extracellular polymeric substance decomposition linked to hydrogen recovery from waste activated sludge: Role of peracetic acid and free nitrous acid co-pretreatment in a prefermentation-bioelectrolysis cascading system. Water Res. 2020, 176, 115724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shang, Y.; Gong, T.; Hu, Z.; Yang, K.; Shao, S. High concentration of Mn2+ has multiple influences on aerobic granular sludge for aniline wastewater treatment. Chemosphere 2020, 240, 124945–124953. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, X.; Huppes, G.; Zhao, X.; Ren, N. Using site-specific life cycle assessment methodology to evaluate Chinese wastewater treatment scenarios: A comparative study of site generic and site-specific methods. J. Clean. Prod. 2017, 144, 1–7. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, A.; Wen, K.; Liu, Z.; Liu, W.; Wang, A.; Yue, X. Upgrading VFAs bioproduction from waste activated sludge via co-fermentation with soy sauce residue. Front. Environ. Sci. Eng. 2019, 13, 3. [Google Scholar] [CrossRef]
- Carranzo, I.V. Standard Methods for examination of water and wastewater. An. De Hidrol. Médica 2012, 5, 185–186. [Google Scholar]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.-Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, X.; Xiao, K.; Shen, N.; Zeng, R.J.; Zhou, Y. Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase—Investigation on dissolved organic matter transformation and microbial community shift. Water Res. 2017, 112, 261–268. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Yin, X.; Li, Q.; Li, X.; Zhang, Y.; Deng, Y. Insights into biomethane production and microbial community succession during semi-continuous anaerobic digestion of waste cooking oil under different organic loading rates. AMB Express 2018, 8, 92. [Google Scholar] [CrossRef]
- Zhang, K.; Song, L.; Dong, X. Proteiniclasticum ruminis gen. nov., sp. nov., a strictly anaerobic proteolytic bacterium isolated from yak rumen. Int. J. Syst. Evol. Microbiol. 2010, 60, 2221–2225. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Marsic, D.; Whitman, W.B.; Lupa, B.; Tang, J.; Krader, P. Proteocatella sphenisci gen. nov., sp. nov., a psychrotolerant, spore-forming anaerobe isolated from penguin guano. Int. J. Syst. Evol. Microbiol. 2009, 5, 2302–2307. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zheng, S.; Wang, D.; Wang, L.; Wang, G. Thermomonas carbonis sp. nov., isolated from the soil of a coal mine. Int. J. Syst. Evol. Microbiol. 2014, 64, 3631–3635. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Koeck, D.E.; Klocke, M. Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and Fermentimonas caenicola gen. nov., sp. nov. isolated from mesophilic laboratory-scale biogas reactors and emended description of the genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 2016, 66, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Huo, X.; Du, W.J.; Liang, J.D.; Wang, D.Q.; Yang, S.C. Biodegradation of kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett. Appl. Microbiol. 2016, 62, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Fuentes, S.; Grievink, W.; Niftrik, L.v.; Tindall, B.J.; Timmerman, H.M.; Rijkers, G.T.; Smidt, H. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1600–1616. [Google Scholar]
- Daneshvar, M.I.; Douglas, M.P.; Weyant, R.S. Cellular fatty acid composition of Lautropia mirabilis. J. Clin. Microbiol. 2001, 39, 4160–4162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.W.; Liu, W.Z.; Gao, Q.; Tang, C.C.; Wang, L.; Guo, Z.C.; Zhou, A.J.; Wang, A.J. Potassium ferrate addition as an alternative pre-treatment to enhance short-chain fatty acids production from waste activated sludge. Bioresour. Technol. 2017, 247, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Garrity, G.M.; Labeda, D.P.; Oren, A. Judicial Commission of the International Committee on Systematics of Prokaryotes XIIth International (IUMS) Congress of Bacteriology and Applied Microbiology. Int. J. Syst. Evol. Microbiol. 2011, 61, 2775–2780. [Google Scholar] [CrossRef]
- Zuo, N.; He, J.; Ma, X.; Peng, Y.; Li, X. Phosphorus removal performance and population structure of phosphorus-accumulating organisms in HA-A/A-MCO sludge reduction process. Bioengineered 2016, 7, 327–333. [Google Scholar] [CrossRef] [Green Version]





| Group | CSCFAs∞ (mg COD/L) | Crate (h) | T50 (h) | R2 | p |
|---|---|---|---|---|---|
| F-7.0 | 1334 ± 36 | 14.87 ± 0.78 | 54.19 ± 0.96 | 0.99 | <0.001 |
| F-10.0 | 2258 ± 41 | 3.22 ± 0.65 | 13.73 ± 2.61 | 0.98 | 0.003 |
| Group | Time (h) | ||||
|---|---|---|---|---|---|
| 0 | 12 | 24 | 120 | 240 | |
| F-7.0 | 11.28 ± 1.2 | 11.05 ± 0.2 | 10.49 ± 0.6 | 9.81 ± 0.7 | 8.46 ± 0.3 |
| F-10.0 | 11.28 ± 1.2 | 10.57 ± 0.5 | 9.52 ± 0.4 | 8.08 ± 1.0 | 6.09 ± 0.1 |
| Groups | VFA Production | Reduced Methanol Addition a | Caustic Soda Addition | PED b | GWP |
|---|---|---|---|---|---|
| kg VFAs-COD/d | kg/d | kg/d | MJ | kg CO2 Eq | |
| F-7.0 | 18.2 | 12.1 | 0.0 | −791.4 | −16.8 |
| F-10.0 | 77.6 | 51.7 | 0.2 | −3371.5 | −71.4 |
| Difference value | 59.4 | 39.6 | 0.2 | −2580.1 | −54.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Zhou, A.; Yue, X.; Zhang, Z.; Gao, Y.; Luo, Y. Intensification of Short Chain Fatty Acid Production during the Alkaline Pretreatment of Fine-Sieving Fractions. Energies 2020, 13, 4690. https://doi.org/10.3390/en13184690
Duan Y, Zhou A, Yue X, Zhang Z, Gao Y, Luo Y. Intensification of Short Chain Fatty Acid Production during the Alkaline Pretreatment of Fine-Sieving Fractions. Energies. 2020; 13(18):4690. https://doi.org/10.3390/en13184690
Chicago/Turabian StyleDuan, Yanqing, Aijuan Zhou, Xiuping Yue, Zhichun Zhang, Yanjuan Gao, and Yanhong Luo. 2020. "Intensification of Short Chain Fatty Acid Production during the Alkaline Pretreatment of Fine-Sieving Fractions" Energies 13, no. 18: 4690. https://doi.org/10.3390/en13184690
APA StyleDuan, Y., Zhou, A., Yue, X., Zhang, Z., Gao, Y., & Luo, Y. (2020). Intensification of Short Chain Fatty Acid Production during the Alkaline Pretreatment of Fine-Sieving Fractions. Energies, 13(18), 4690. https://doi.org/10.3390/en13184690