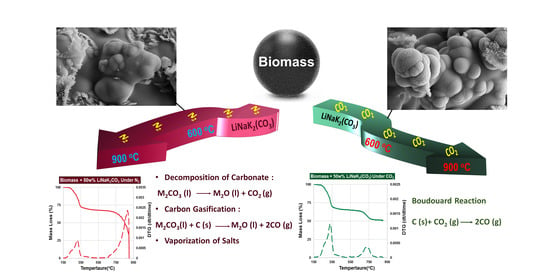
Thermochemical Conversion of Biomass in the Presence of Molten Alkali-Metal Carbonates under Reducing Environments of N2 and CO2
Abstract
:1. Introduction
2. Materials and Methodology
3. Results
3.1. Thermochemical Conversion under N2 vs. CO2 at Low HHT (600 °C)
3.2. Thermochemical Conversion under N2 vs. CO2 at High HHT (900 °C)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lahijani, P.; Mohammadi, M.; Zainal, Z.A.; Mohamed, A. Advances in CO2 gasification reactivity of biomass char through utilization of radio frequency irradiation. Energy 2015, 93, 976–983. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M. Catalytic Effect of Metal Species on Enhancement of CO2 Gasification Reactivity of Biomass Char. Int. J. Eng. 2015, 28, 1251–1256. [Google Scholar]
- Nunes, L.; Matias, J.; Catalão, J. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total. Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Chhiti, Y.; Ouladsine, R.; Sahibeddine, A.; Bensitel, M. Catalytic effect of ash on bio-oil thermal conversion. Moroc. J. Chem. 2016, 4, 584–591. [Google Scholar]
- Jalalabadi, T.; Li, C.; Yi, H.; Lee, D. A TGA study of CO2 gasification reaction of various types of coal and biomass. J. Mech. Sci. Technol. 2016, 30, 3275–3281. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Vaárhegyi, G.; Skreiberg, Ø.; Løvås, T. CO2 Gasification of Chars Prepared by Fast and Slow Pyrolysis from Wood and Forest Residue: A Kinetic Study. Energy Fuels 2018, 32, 588–597. [Google Scholar] [CrossRef]
- Russell, S.H.; Turrion-Gomez, J.L.; Meredith, W.; Langston, P.A.; Snape, C. Increased charcoal yield and production of lighter oils from the slow pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2017, 124, 536–541. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrog. Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Chhiti, Y.; Kemiha, M. Thermal conversion of biomass, pyrolysis and gasification. Int. J. Eng. Sci. (IJES) 2013, 2, 75–85. [Google Scholar]
- Belgaum, V. Tar formation, Reduction and Technology of Tar during Biomass Gasification/Pyrolysis—An Overview. Int. J. Eng. Res. Technol. 2017, 6. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Masnadi, M.S.; Grace, J.R.; Bi, X.T.; Lim, C.J.; Ellis, N. From fossil fuels towards renewables: Inhibitory and catalytic effects on carbon thermochemical conversion during co-gasification of biomass with fossil fuels. Appl. Energy 2015, 140, 196–209. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; González-García, C.M.; Nabais, J.M.V.; Ortiz, A.L. Porosity Development in Activated Carbons Prepared from Walnut Shells by Carbon Dioxide or Steam Activation. Ind. Eng. Chem. Res. 2009, 48, 7474–7481. [Google Scholar] [CrossRef]
- Dong, S.; He, X.; Zhang, H.; Xie, X.; Yu, M.; Yu, C.; Xiao, N.; Qiu, J. Surface modification of biomass-derived hard carbon by grafting porous carbon nanosheets for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15954–15960. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, C.; Zhu, S.; Bai, Y.; Li, F. Tar formation during coal pyrolysis under N2 and CO2 atmospheres at elevated pressures. J. Anal. Appl. Pyrolysis 2016, 118, 130–135. [Google Scholar] [CrossRef]
- Jalalabadi, T.; Glenn, M.; Tremain, P.; Moghtaderi, B.; Donne, S.W.; Allen, J. Modification of Biochar Formation during Slow Pyrolysis in the Presence of Alkali Metal Carbonate Additives. Energy Fuels 2019, 33, 11235–11245. [Google Scholar] [CrossRef]
- Umeki, K.; Häggström, G.; Bach-Oller, A.; Kirtania, K.; Furusjö, E. Reduction of Tar and Soot Formation from Entrained-Flow Gasification of Woody Biomass by Alkali Impregnation. Energy Fuels 2017, 31, 5104–5110. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Rahman, M.; Gupta, R.; Mims, C.A.; Hill, J.M. K2CO3 catalyzed CO2 gasification of ash-free coal. Interactions of the catalyst with carbon in N2 and CO2 atmosphere. Fuel 2014, 117, 1181–1189. [Google Scholar] [CrossRef]
- Bach-Oller, A.; Fursujo, E.; Umeki, K. Effect of potassium impregnation on the emission of tar and soot from biomass gasification. Energy Procedia 2019, 158, 619–624. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Larsen, F.H.; Shchukarev, A.; Ståhl, K.; Umeki, K. Potassium and soot interaction in fast biomass pyrolysis at high temperatures. Fuel 2018, 225, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Lua, A.C. Textural and chemical characterizations of adsorbent prepared from palm shell by potassium hydroxide impregnation at different stages. J. Colloid Interface Sci. 2002, 254, 227–233. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Sapag, K.; Rodríguez-Reinoso, F. Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO2. Carbon 2016, 110, 138–147. [Google Scholar] [CrossRef]
- Rutkowski, P. Pyrolysis of cellulose, xylan and lignin with the K2CO3 and ZnCl2 addition for bio-oil production. Fuel Process. Technol. 2011, 92, 517–522. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Heuer, S.; Ammendola, P. Slow pyrolysis of walnut shells in nitrogen and carbon dioxide. Fuel 2018, 225, 419–425. [Google Scholar] [CrossRef]
- Fu, P.; Yi, W.; Li, Z.; Bai, X.; Wang, L. Evolution of char structural features during fast pyrolysis of corn straw with solid heat carriers in a novel V-shaped down tube reactor. Energy Convers. Manag. 2017, 149, 570–578. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.I.; Vithanage, M.; Park, Y.K.; Lee, J.; Kwon, E.E.; Ok, J.I. Modification of biochar properties using CO2. Chem. Eng. J. 2019, 372, 383–389. [Google Scholar] [CrossRef]
- Kirtania, K.; Axelsson, J.; Matsakas, L.; Christakopoulos, P.; Umeki, K.; Furusjö, E. Kinetic study of catalytic gasification of wood char impregnated with different alkali salts. Energy 2017, 118, 1055–1065. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Davidson, J.H.; Kittelson, D.B. Solar Gasification of Biomass: Kinetics of Pyrolysis and Steam Gasification in Molten Salt. J. Sol. Energy Eng. 2011, 133, 021011. [Google Scholar] [CrossRef]
- McKee, D.W. Catalytic effects of alkaline earth carbonates in the carbon-carbon dioxide reaction. Fuel 1980, 59, 308–314. [Google Scholar] [CrossRef]
- Olivares, R.I.; Chen, C.; Wright, S. The Thermal Stability of Molten Lithium–Sodium–Potassium Carbonate and the Influence of Additives on the Melting Point. J. Sol. Energy Eng. 2012, 134, 041002. [Google Scholar] [CrossRef]
- Glenn, M.J.; Allen, J.; Donne, S.W. Thermal Investigation of a Doped Alkali-Metal Carbonate Ternary Eutectic for Direct Carbon Fuel Cell Applications. Energy Fuels 2015, 29, 5423–5433. [Google Scholar] [CrossRef]
- Glenn, M.; Mathan, B.; Islam, M.; Beyad, Y.; Allen, J.A.; Donne, S.W. Gas Atmosphere Effects Over the Anode Compartment of a Tubular Direct Carbon Fuel Cell Module. Energy Fuels 2019, 33, 7901–7907. [Google Scholar] [CrossRef]
- Fereres, S.; Prieto, C.; Gavarrell, P.G.; Rodríguez, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Molten carbonate salts for advanced solar thermal energy power plants: Cover gas effect on fluid thermal stability. Sol. Energy Mater. Sol. Cells 2018, 188, 119–126. [Google Scholar] [CrossRef]
- Glenn, M.J.; Allen, J.A.; Donne, S.W. Carbon Gasification from a Molten Carbonate Eutectic. Energy Technol. 2019, 7. [Google Scholar] [CrossRef]
- Klopper, L.; Strydom, C.A.; Bunt, J.R. Influence of added potassium and sodium carbonates on CO2 reactivity of the char from a demineralized inertinite rich bituminous coal. J. Anal. Appl. Pyrolysis 2012, 96, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Hughes, M.A.; Allen, J.; Donne, S.W. The properties and performance of carbon produced through the electrochemical reduction of molten carbonate: A study based on step potential electrochemical spectroscopy. Electrochim. Acta 2018, 278, 340–351. [Google Scholar] [CrossRef]
- Li, C.; Yi, H.; Jalalabadi, T.; Lee, D. Thermal decomposition of alkane hydrocarbons inside a porous Ni anode for fuel supply of direct carbon fuel cell: Effects of morphology and crystallinity of carbon. J. Power Sources 2015, 294, 284–291. [Google Scholar] [CrossRef]
- Peng, F.; Li, Y.; Nash, P.; Cooper, J.F.; Parulekar, S.J.; Selman, J.R. Direct Carbon Fuel Cells—Wetting behavior of graphitic carbon in molten carbonate. Int. J. Hydrog. Energy 2016, 41, 18858–18871. [Google Scholar] [CrossRef]
- Yoshida, S.; Matsunami, J.; Hosokawa, Y.; Yokota, O.; Tamaura, Y.; Kitamura, M. Coal/CO2 Gasification System Using Molten Carbonate Salt for Solar/Fossil Energy Hybridization. Energy Fuels 1999, 13, 961–964. [Google Scholar] [CrossRef]
- Yeboah, Y.D.; Xu, Y.; Sheth, A.; Godavarty, A.; Agrawal, P.K. Catalytic gasification of coal using eutectic salts: Identification of eutectics. Carbon 2003, 41, 203–214. [Google Scholar] [CrossRef]
- Claes, P.; Moyaux, D.; Peeters, D. Solubility and solvation of carbon dioxide in the molten Li2CO3/Na2CO3/K2CO3 (43.5:31.5:25.0 mol-%) eutectic mixture at 973 K I. Experimental part. Eur. J. Inorg. Chem. 1999, 1999, 583–588. [Google Scholar] [CrossRef]
- Jalalabadi, T.; Drewery, M.; Tremain, P.; Wilkinson, J.; Moghtaderi, B.; Allen, J. The Impact of Carbonate Salts on Char and Gaseous Evolution during Slow Pyrolysis of Biomass, Cellulose, and Lignin. Sustain. Energy Fuels 2020. [Google Scholar] [CrossRef]
- Nygård, H.S.; Olsen, E. Molten salt pyrolysis of milled beech wood using an electrostatic precipitator for oil collection. AIMS Energy 2015, 3, 284–296. [Google Scholar] [CrossRef]
- Su, S.; Song, Y.; Wang, Y.; Li, T.; Hu, S.; Xiang, J.; Li, C.Z. Effects of CO2 and heating rate on the characteristics of chars prepared in CO2 and N2 atmospheres. Fuel 2015, 142, 243–249. [Google Scholar] [CrossRef]
- Khan, M.R.; Jenkins, R.G. Influence of Added Calcium Compounds on Swelling, Plastic, and Pyrolysis Behavior of Coal Devolatilized at Elevated Pressures. Fuel 1986, 65, 1203–1208. [Google Scholar] [CrossRef]
- Khan, M.; Jenkins, R.G. Swelling and plastic properties of coal devolatilized at elevated pressures: An examination of the influences of coal type. Fuel 1986, 65, 725–731. [Google Scholar] [CrossRef]
- Riaza, J.; Ajmi, M.; Gibbins, J.; Chalmers, H. Ignition and combustion of single particles of coal and biomass under O2/CO2 atmospheres. In Proceedings of the 13th International Conference on Greenhouse Gas Control Technologies, Ghgt-13, Lausanne, Switzerland, 14–18 November 2016; 2017; Volume 114, pp. 6067–6073. [Google Scholar]
- Riaza, J.; Khatami, R.; Levendis, Y.A.; Álvarez, L.; Gil, M.V.; Pevida, C.; Rubiera, F.; Pis, J.J. Combustion of single biomass particles in air and in oxy-fuel conditions. Biomass Bioenergy 2014, 64, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Perander, M.; DeMartini, N.; Brink, A.; Kramb, J.; Karlström, O.; Hemming, J.; Moilanen, A.; Konttinen, J.; Hupa, M. Catalytic effect of Ca and K on CO2 gasification of spruce wood char. Fuel 2015, 150, 464–472. [Google Scholar] [CrossRef]
- Mims, C. Catalytic Gasification of Carbon: Fundamentals and Mechanism, in Fundamental Issues in Control of Carbon Gasification Reactivity; Springer: Berlin/Heidelberg, Germany, 1991; pp. 383–407. [Google Scholar]
- McKee, D.; Chatterji, D. The catalytic behavior of alkali metal carbonates and oxides in graphite oxidation reactions. Carbon 1975, 13, 381–390. [Google Scholar] [CrossRef]
- An, W.; Sun, X.; Jiao, Y.; Wang, S.; Wang, W.; Tadé, M.O.; Shao, Z.; Li, S.D.; Shuang, S. Inherently Catalyzed Boudouard Reaction of Bamboo Biochar for Solid Oxide Fuel Cells with Improved Performance. Energy Fuels 2018, 32, 4559–4568. [Google Scholar] [CrossRef]









| Number | Reaction | ΔHoR |
|---|---|---|
| Oxidation Reactions | ||
| 1 | C + ½ O2 → CO | −111 (MJ/kmol) |
| 2 | CO+½ O2 → CO2 | −283 (MJ/kmol) |
| 3 | H2 + ½ O2 → H2O | −242 (MJ/kmol) |
| Gasification Reactions | ||
| 4 | C + H2O → CO + H2 (Water-Gas reaction) | +131 (MJ/kmol) |
| 5 | C + CO2 → 2CO (Boudouard reaction) | +172 (MJ/kmol) |
| 6 | C + 2H2 → CH4 (Methanation reaction) | −75 (MJ/kmol) |
| 7 | CO + H2O → CO2 + H2 (Water–Gas–Shift reaction) | −41 (MJ/kmol) |
| 8 | CH4 + H2O → CO + 3H2 (Methane–Steam reforming) | +206 (MJ/kmol) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalalabadi, T.; Moghtaderi, B.; Allen, J. Thermochemical Conversion of Biomass in the Presence of Molten Alkali-Metal Carbonates under Reducing Environments of N2 and CO2. Energies 2020, 13, 5395. https://doi.org/10.3390/en13205395
Jalalabadi T, Moghtaderi B, Allen J. Thermochemical Conversion of Biomass in the Presence of Molten Alkali-Metal Carbonates under Reducing Environments of N2 and CO2. Energies. 2020; 13(20):5395. https://doi.org/10.3390/en13205395
Chicago/Turabian StyleJalalabadi, Tahereh, Behdad Moghtaderi, and Jessica Allen. 2020. "Thermochemical Conversion of Biomass in the Presence of Molten Alkali-Metal Carbonates under Reducing Environments of N2 and CO2" Energies 13, no. 20: 5395. https://doi.org/10.3390/en13205395
APA StyleJalalabadi, T., Moghtaderi, B., & Allen, J. (2020). Thermochemical Conversion of Biomass in the Presence of Molten Alkali-Metal Carbonates under Reducing Environments of N2 and CO2. Energies, 13(20), 5395. https://doi.org/10.3390/en13205395