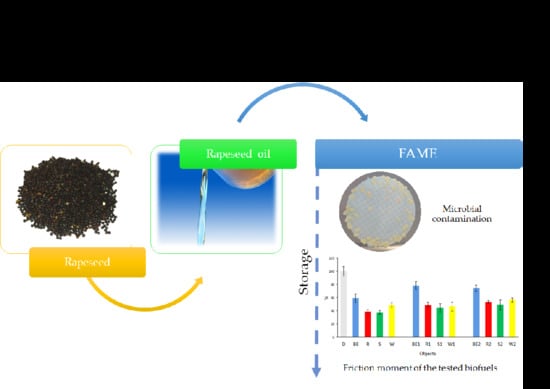
Functional Properties and Microbiological Stability of Fatty Acid Methyl Esters (FAME) under Different Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing and Analysis Procedure
2.3. Statistical Analysis
3. Results and Discussion
3.1. Microbial Contamination of Biofuels
3.2. Acidity of Biofuels
3.3. Dynamic Viscosity and Tribological Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef]
- Rasool, U.; Hemalatha, S. A review on bioenergy and biofuels: Sources and their production. Braz. J. Biol. Sci. 2016, 3, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.P.; Roy, P.S.; Kim, S.H. Current developments in thermochemical conversion of biomass to fuels and chemicals. IntechOpen 2018. [Google Scholar] [CrossRef]
- Sharma, S.; Meena, R.; Sharma, A.; Goyal, P.K. Biomass conversion technologies for renewable energy and fuels: A Review note. IOSR J. Mech. Civ. Eng. 2014, 11, 28–35. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energ. Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.-Y.; Liu, D.-H.; Li, Z.-B. Study on acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil biodiesel production. J. Mol. Catal. B Enzym. 2005, 37, 68–71. [Google Scholar] [CrossRef]
- Pleanjai, S.; Gheewala, S.H.; Garivait, S. Environmental evaluation of biodiesel production from palm oil in a life cycle perspective. Asian J. Energy Environ. 2007, 8, 15–32. [Google Scholar]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Biores. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef]
- Porte, A.V.; Schneider, R.C.S.; Kaercher, J.A.; Klamt, R.A.; Schmatz, W.L.; Da Silva, W.L.T.; Filho, W.A.S. Sunflower biodiesel production and application in family farms in Brazil. Fuel 2010, 89, 3718–3724. [Google Scholar] [CrossRef]
- Yoo, S.J.; Veriansyah, B.; Kim, J.; Kim, J.D.; Lee, Y.W. Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalyst. Biores. Technol. 2010, 101, 8686–8689. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M.; Sanli, H. Biodiesel production from vegetable oil and waste animal fats in a pilot plant. Waste Manag. 2014, 34, 2146–2154. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenkovic, O.S.; Veljkovic, V.B.; Hung, Y.-T. Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S. A review on biodiesel production technique. Eng. J. Appl. Scopes 2018, 3, 5–8. [Google Scholar]
- Leung, D.Y.C.; Koo, B.C.P.; Guo, Y. Degradation of biodiesel under different storage conditions. Biores. Technol. 2006, 97, 250–256. [Google Scholar] [CrossRef]
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Patil, P.D.; Mulla, M.Z. Review on different sources for the production of biodiesel. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2017, 6, 21–25. [Google Scholar]
- Komariah, L.N.; Aprisah, M.S.; Rosa, Y.S.L. Storage tank materials for biodiesel blends; the analysis of fuel property changes. MATEC Web Conf. 2017, 101, 02012. [Google Scholar] [CrossRef] [Green Version]
- Hawrot-Paw, M.; Smolik, B.; Kamieniecka, A. Preliminary study of the efficiency of biodiesel biological decomposition with autochthonous soil microflora. Ecol. Chem. Eng. 2011, 18, 1401–1406. [Google Scholar]
- Fregolente, P.B.L.; Fregolente, L.V.; Maciel, M.R.W. Water content in biodiesel, diesel, and biodiesel–diesel blends. J. Chem. Eng. Data. 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- Siegert, W. Microbial Contamination in Diesel Fuel—Are New Problems Arising from Biodiesel Blends? In Proceedings of the 11th International Conference on Stability, Handling and Use of Liquid Fuels, Prague, Czech Republic, 18–22 October 2009; p. 3. [Google Scholar]
- Klofutar, B.; Golob, J. Microorganisms in diesel and in biodiesel fuels. Acta Chim. Slov. 2007, 54, 744–758. [Google Scholar]
- Zakaria, H.; Khalid, A.; Sies, M.F.; Mustaffa, N.; Manshoor, B. Effect of storage temperature and storage duration on Biodiesel properties and characteristics. Appl. Mech. Mater. 2014, 465–466, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Malarvizhi, S.; Krishnamurthy, S.R. Microbiologically influenced corrosion of carbon steel exposed to biodiesel. Int. J. Corros. 2016. [Google Scholar] [CrossRef]
- Dodos, G.S.; Konstantakos, T.; Longinos, S.; Zannikos, F. Effects of microbiological contamination in the quality of biodiesel fuels. Global Nest J. 2012, 14, 175–182. [Google Scholar]
- Biernat, K. Biofuels in storage and operating conditions. In Storage Stability of Fuels; IntechOpen: London, UK, 2015. [Google Scholar]
- Sørensen, G.; Pedersen, D.V.; Norgaard, A.K.; Sorensen, K.B.; Nygaard, S.D. Microbial growth studies in biodiesel blends. Bioresour. Technol. 2011, 102, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Tamaldin, N.; Jaat, M.; Ali, M.F.M.; Manshoor, B.; Zaman, I. Impacts of biodiesel storage duration on fuel properties and emissions. Proc. Eng. 2013, 68, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, P.; Borugadda, V.B.; Goud, V.V.; Sahoo, L. Effect of storage parameters on stability of Jatropha-derived biodiesel. Int. J. Energy Environ. Eng. 2013, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Christensen, E.; McCormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, T.; Werkmeister, R.; Russ, W.; Meyer-Pittroff, R. Microbiological stability of biodiesel–diesel-mixtures. Bioresour. Technol. 2009, 100, 724–730. [Google Scholar] [CrossRef]
- Bücker, F.; Santestevan, N.A.; Roesch, L.F.; Jacques, R.J.S.; Peralba, M.C.R.; Camargo, F.A.O.; Bento, F.M. Impact of biodiesel on biodeterioration of stored Brazilian diesel fuel. Int. Biodeterior. Biodegrad. 2011, 65, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.C.; Hill, G.C. Strategies for Resolving Problems Caused by Microbial Growth in Terminals and Retail Sites Handling Biodiesels. In Proceedings of the 11th International Conference on Stability, Handling and Use of Liquid Fuels, Prague, Czech Republic, 18–22 October 2009; Available online: http://toc.proceedings.com/07515webtoc.pdf (accessed on 14 October 2020).
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Stamps, B.W.; Bojanowski, C.L.; Drake, C.A.; Nunn, H.S.; Lloyd, P.F.; Floyd, J.G.; Emmerrich, K.A.; Neal, A.R.; Crookes-Goodson, W.J.; Stevenson, B.S. In situ linkage of fungal and bacterial proliferation to microbiologically influenced corrosion in B20 biodiesel storage tanks. Front. Microbiol. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luz, G.; Sousa, B.; Guedes, A.; Barreto, C.; Brasil, L. Biocides used as additives to biodiesels and their risks to the environment and public health: A review. Molecules 2018, 23, 2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci. Rep. 2020, 10, 16436. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, F.B.; Sobral, S.P. A new method for determining the acid number of biodiesel based on coulometric titration. Talanta 2012, 97, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Das, L.M.; Bora, D.K.; Pradhan, S.; Naik, M.K.; Naik, S.N. Long-term storage stability of biodiesel produced from Karanja oil. Fuel 2009, 88, 2315–2318. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Investigation of friction and wear characteristics of palm biodiesel. Energ. Convers. Manag. 2013, 67, 251–256. [Google Scholar] [CrossRef]
- Uchôa, I.M.A.; Neto, A.A.D.; Da Silva, S.E.; De Lima, L.F.; De Barros, N.E.L. Evaluation of Lubricating Properties of Diesel Based Fuels Micro Emulsified with Glycerin. Mater. Res. 2017, 20, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Sukjit, E.; Tongroon, M.; Chollacoop, N.; Yoshimura, Y.; Poapongsakorn, P.; Lapuerta, M.; Dearn, K.D. Improvement of the tribological behavior of palm biodiesel via partial hydrogenation of unsaturated fatty acid methyl esters. Wear 2019, 426–427 Pt A, 813–818. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Chong, W.W.F.; Ng, J.-H.; Ghazali, M.J.; Wood, R.J.K. Influence of fatty acid methyl ester composition on tribological properties of vegetable oils and duck fat derived biodiesel. Tribol. Int. 2017, 113, 76–82. [Google Scholar] [CrossRef]









| Storage conditions | Rapeseed Methyl Ester | Sunflower Methyl Ester | Waste Methyl Ester | Bioester | Diesel |
|---|---|---|---|---|---|
| no storage, initial stage | R | S | W | BE | D |
| outside, ambient temperature (1) | R1 | S1 | W1 | BE1 | - |
| inside (refrigerator), 4 °C (2) | R2 | S2 | W2 | BE2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Koniuszy, A.; Sędłak, P.; Seń, D. Functional Properties and Microbiological Stability of Fatty Acid Methyl Esters (FAME) under Different Storage Conditions. Energies 2020, 13, 5632. https://doi.org/10.3390/en13215632
Hawrot-Paw M, Koniuszy A, Sędłak P, Seń D. Functional Properties and Microbiological Stability of Fatty Acid Methyl Esters (FAME) under Different Storage Conditions. Energies. 2020; 13(21):5632. https://doi.org/10.3390/en13215632
Chicago/Turabian StyleHawrot-Paw, Małgorzata, Adam Koniuszy, Paweł Sędłak, and Daria Seń. 2020. "Functional Properties and Microbiological Stability of Fatty Acid Methyl Esters (FAME) under Different Storage Conditions" Energies 13, no. 21: 5632. https://doi.org/10.3390/en13215632
APA StyleHawrot-Paw, M., Koniuszy, A., Sędłak, P., & Seń, D. (2020). Functional Properties and Microbiological Stability of Fatty Acid Methyl Esters (FAME) under Different Storage Conditions. Energies, 13(21), 5632. https://doi.org/10.3390/en13215632