Oriented Fermentation of Food Waste towards High-Value Products: A Review
Abstract
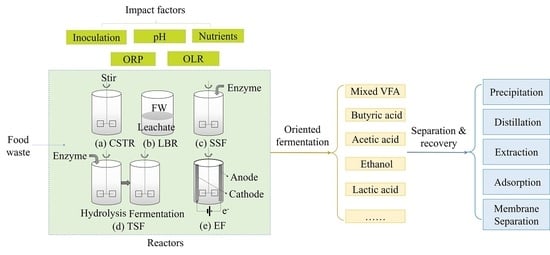
:1. Introduction
2. Basic Principles and Impact Factors
2.1. Inoculation
2.2. Metabolism
2.3. pH
2.4. Oxidoreduction Potential
2.5. Organic Loading Rate
2.6. Nutrients
3. Fermentation Types and Products
3.1. Fermentation towards Mixed VFA Production
3.2. Fermentation towards Butyric Acid Production
3.3. Fermentation towards Acetic Acid Production
3.4. Fermentation towards Ethanol Production
3.5. Fermentation towards Lactic Acid Production
3.6. Fermentation towards Other Products
4. Processes and Reactors
4.1. Continuously Stirred Tank Reactors
4.2. Solid–Liquid Separation Reactors
4.3. Single-Stage and Two-Stage Fermentation
4.4. Electro-Fermentation
4.5. Additive Materials
5. Products Separation and Recovery
5.1. Precipitation
5.2. Distillation
5.3. Extraction
5.4. Adsorption
5.5. Membrane Separation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.B.D.; Kumar, G.; Lin, C.-Y. An overview of food waste management in developing countries: Current status and future perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, A.; Jaffar, M.; Li, X. Improving biomethane yield by controlling fermentation type of acidogenic phase in two-phase anaerobic co-digestion of food waste and rice straw. Chem. Eng. J. 2015, 273, 254–260. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Zhang, L.; Jahng, D. Long-term anaerobic digestion of food waste stabilized by trace elements. Waste Manag. 2012, 32, 1509–1515. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-k.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Chu, C.-F.; Li, Y.-Y.; Xu, K.-Q.; Ebie, Y.; Inamori, Y.; Kong, H.-N. A pH- and temperature-phased two-stage process for hydrogen and methane production from food waste. Int. J. Hydrogen Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, R.; El-Mashad, H.M.; Sun, H.; Ying, Y. Effect of food to microorganism ratio on biohydrogen production from food waste via anaerobic fermentation. Int. J. Hydrogen Energy 2008, 33, 6968–6975. [Google Scholar] [CrossRef]
- Browne, J.D.; Murphy, J.D. Assessment of the resource associated with biomethane from food waste. Appl. Energy 2013, 104, 170–177. [Google Scholar] [CrossRef]
- Iacovidou, E.; Ohandja, D.-G.; Gronow, J.; Voulvoulis, N. The Household Use of Food Waste Disposal Units as a Waste Management Option: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1485–1508. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Merrylin, J.; Devi, T.P.; Kavitha, S.; Sivashanmugam, P.; Kumar, G.; Banu, J.R. Food waste valorization: Biofuels and value added product recovery. Bioresour. Technol. Rep. 2020, 11, 100524. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Imbeah, M. Composting piggery waste: A review. Bioresour. Technol. 1998, 63, 197–203. [Google Scholar] [CrossRef]
- Cheng, J.Y.K.; Lo, I.M.C. Investigation of the available technologies and their feasibility for the conversion of food waste into fish feed in Hong Kong. Environ. Sci. Pollut. Res. 2016, 23, 7169–7177. [Google Scholar] [CrossRef] [PubMed]
- Kwak, W.S.; Kang, J.S. Effect of feeding food waste-broiler litter and bakery by-product mixture to pigs. Bioresour. Technol. 2006, 97, 243–249. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.-H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Paluszak, Z.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Pińkowska, H. Pathogen Reduction Potential in Anaerobic Digestion of Organic Fraction of Municipal Solid Waste and Food Waste. Molecules 2020, 25, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, D.; Wen, Z.; Gottfried, O.; Schmidt, F.; Fei, F. A review of global strategies promoting the conversion of food waste to bioenergy via anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 79, 204–221. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Anwar Saeed, M.; Ma, H.; Yue, S.; Wang, Q.; Tu, M. Concise review on ethanol production from food waste: development and sustainability. Environ. Sci. Pollut. Res. 2018, 25, 28851–28863. [Google Scholar] [CrossRef]
- Jeffries, T.W.; Jin, Y.S. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol. 2004, 63, 495–509. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fei, Q.; Zhang, Y.; Contreras, L.M.; Utturkar, S.M.; Brown, S.D.; Himmel, M.E.; Zhang, M. Zymomonas mobilis as a model system for production of biofuels and biochemicals. Microb. Biotechnol. 2016, 9, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Park, J.M.; Kim, T.Y.; Yun, H.; Lee, S.Y. The genome-scale metabolic network analysis of Zymomonas mobilis ZM4 explains physiological features and suggests ethanol and succinic acid production strategies. Microb. Cell Factories 2010, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic bacterial lactic acid fermentation—A review. Biotechnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.d.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Im, J.A.; Choi, S.Y.; Lee, J.I.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab. Eng. 2014, 23, 165–174. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Lee, J.Y.; Lee, J.; Park, J.H.; Im, J.A.; Eom, M.-H.; Lee, J.; Lee, S.-H.; Song, H.; Cho, J.-H.; et al. Enhanced Butanol Production Obtained by Reinforcing the Direct Butanol-Forming Route in Clostridium acetobutylicum. mBio 2012, 3, e00312–e00314. [Google Scholar] [CrossRef] [Green Version]
- Zigová, J.; Šturdı́k, E.; Vandák, D.; Schlosser, Š. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 1999, 34, 835–843. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Coats, E.R.; Gregg, M.; Crawford, R.L. Effect of organic loading and retention time on dairy manure fermentation. Bioresour. Technol. 2011, 102, 2572–2577. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Temudo, M.F.; Kleerebezem, R.; van Loosdrecht, M. Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.-S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Bi, S.; Hong, X.J.; Wang, G.X.; Li, Y.; Gao, Y.M.; Yan, L.; Wang, Y.J.; Wang, W.D. Effect of domestication on microorganism diversity and anaerobic digestion of food waste. Genet. Mol. Res. 2016, 15, 1–14. [Google Scholar] [CrossRef]
- Maamri, S.; Amrani, M. Biogas Production from Waste Activated Sludge Using Cattle Dung Inoculums: Effect of Total Solid Contents and Kinetics Study. Energy Procedia 2014, 50, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhao, J.; Xu, Q.; Li, X.; Wang, D.; Yang, Q.; Liu, Y.; Tao, Z. Enhanced volatile fatty acids production from waste activated sludge anaerobic fermentation by adding tofu residue. Bioresour. Technol. 2019, 274, 430–438. [Google Scholar] [CrossRef]
- Romano, A.H.; Conway, T. Evolution of carbohydrate metabolic pathways. Res. Microbiol. 1996, 147, 448–455. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.-Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Ramsay, I.R.; Pullammanappallil, P.C. Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation 2001, 12, 247–256. [Google Scholar] [CrossRef]
- Shen, D.; Yin, J.; Yu, X.; Wang, M.; Long, Y.; Shentu, J.; Chen, T. Acidogenic fermentation characteristics of different types of protein-rich substrates in food waste to produce volatile fatty acids. Bioresour. Technol. 2017, 227, 125–132. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Vidal, G.; Carvalho, A.; Méndez, R.; Lema, J.M. Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour. Technol. 2000, 74, 231–239. [Google Scholar] [CrossRef]
- Mackie, R.I.; White, B.A.; Bryant, M.P. Lipid Metabolism in Anaerobic Ecosystems. Crit. Rev. Microbiol. 1991, 17, 449–479. [Google Scholar] [CrossRef] [PubMed]
- Chipasa, K.B.; Mędrzycka, K. Behavior of lipids in biological wastewater treatment processes. J. Ind. Microbiol. Biotechnol. 2006, 33, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Fu, H.; Ren, M.; Liao, Z.; Ma, Y.; Wang, J. Enhanced butyric acid production in Clostridium tyrobutyricum by overexpression of rate-limiting enzymes in the Embden-Meyerhof-Parnas pathway. J. Biotechnol. 2018, 272–273, 14–21. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.-T. Butyric acid: Applications and recent advances in its bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef]
- Weimer, P.J.; Moen, G.N. Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microbiol. Biotechnol. 2013, 97, 4075–4081. [Google Scholar] [CrossRef]
- Årsköld, E.; Lohmeier-Vogel, E.; Cao, R.; Roos, S.; Rådström, P.; van Niel, E.W.J. Phosphoketolase Pathway Dominates in Lactobacillus reuteri ATCC 55730 Containing Dual Pathways for Glycolysis. J. Bacteriol. 2008, 190, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgé, G.; Saulou-Bérion, C.; Moussa, M.; Allais, F.; Athes, V.; Spinnler, H.-E. Relationships between the use of Embden Meyerhof pathway (EMP) or Phosphoketolase pathway (PKP) and lactate production capabilities of diverse Lactobacillus reuteri strains. J. Microbiol. 2015, 53, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Shinto, H.; Tashiro, Y.; Kobayashi, G.; Sekiguchi, T.; Hanai, T.; Kuriya, Y.; Okamoto, M.; Sonomoto, K. Kinetic study of substrate dependency for higher butanol production in acetone–butanol–ethanol fermentation. Process Biochem. 2008, 43, 1452–1461. [Google Scholar] [CrossRef]
- Zheng, J.; Tashiro, Y.; Wang, Q.; Sonomoto, K. Recent advances to improve fermentative butanol production: Genetic engineering and fermentation technology. J. Biosci. Bioeng. 2015, 119, 1–9. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, J.; Zhang, X. Systematic engineering of pentose phosphate pathway improves Escherichia coli succinate production. Biotechnol. Biofuels 2016, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Matsakas, L.; Christakopoulos, P. Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site. Sustainability 2015, 7, 1446–1458. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.C.; Pak, D. Feasibility of producing ethanol from food waste. Waste Manag. 2011, 31, 2121–2125. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, Y.G.; Kim, G.D.; Kim, S.H.; Chung, T.H. Effect of enzymatic pretreatment on solubilization and volatile fatty acid production in fermentation of food waste. Water Sci. Technol. 2005, 52, 51–59. [Google Scholar] [CrossRef]
- Hashemi, M.; Mousavi, S.M.; Razavi, S.H.; Shojaosadati, S.A. Comparison of submerged and solid state fermentation systems effects on the catalytic activity of Bacillus sp. KR-8104 α-amylase at different pH and temperatures. Ind. Crops Prod. 2013, 43, 661–667. [Google Scholar] [CrossRef]
- Bessler, C.; Schmitt, J.; Maurer, K.-H.; Schmid, R.D. Directed evolution of a bacterial α-amylase: Toward enhanced pH-performance and higher specific activity. Protein Sci. 2003, 12, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Saleemuddin, M. Alkaline proteases: A review. Bioresour. Technol. 1998, 64, 175–183. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, M. An overview of Current and novel approaches for microbial neutral protease improvement. Int. J. Mod. Biol. Med. 2012, 2, 1–27. [Google Scholar]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and Biotechnological Aspects of Microbial Proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Sharma, B.; Shukla, A.K. Biotechnological Approach of Microbial Lipase: A review. Biotechnology 2011, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Li, H.; Zheng, C. Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Zheng, M.; Wang, K. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour. Technol. 2015, 191, 53–58. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xue, C.; Lin, Y.-H.; Bai, F.-W. Redox potential control and applications in microaerobic and anaerobic fermentations. Biotechnol. Adv. 2013, 31, 257–265. [Google Scholar] [CrossRef]
- Zhuge, X.; Li, J.; Shin, H.-d.; Liu, L.; Du, G.; Chen, J. Improved propionic acid production with metabolically engineered Propionibacterium jensenii by an oxidoreduction potential-shift control strategy. Bioresour. Technol. 2015, 175, 606–612. [Google Scholar] [CrossRef]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.M.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sustain. Energy Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Valentino, F.; Pavan, P.; Majone, M.; Bolzonella, D. Optimization of urban waste fermentation for volatile fatty acids production. Waste Manag. 2019, 92, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Various additives for improving dark fermentative hydrogen production: A review. Renew. Sustain. Energy Rev. 2018, 95, 130–146. [Google Scholar]
- Voelklein, M.A.; O’ Shea, R.; Jacob, A.; Murphy, J.D. Role of trace elements in single and two-stage digestion of food waste at high organic loading rates. Energy 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wu, S.; Guo, J.; Zhou, J.; Dong, R. Performance and kinetic evaluation of semi-continuously fed anaerobic digesters treating food waste: Role of trace elements. Bioresour. Technol. 2015, 178, 297–305. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Li, A.; Zhang, L.; Li, R.; Yang, T.; Xing, W. Mechanism of process imbalance of long-term anaerobic digestion of food waste and role of trace elements in maintaining anaerobic process stability. Bioresour. Technol. 2019, 275, 172–182. [Google Scholar] [CrossRef]
- Ezebuiro, N.C.; Körner, I. Characterisation of anaerobic digestion substrates regarding trace elements and determination of the influence of trace elements on the hydrolysis and acidification phases during the methanisation of a maize silage-based feedstock. J. Environ. Chem. Eng. 2017, 5, 341–351. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gomec, C.Y.; Ahn, Y.; Speece, R.E. Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ. Technol. 2003, 24, 1183–1190. [Google Scholar] [CrossRef]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Bioresour. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef]
- Lim, S.-J.; Kim, B.J.; Jeong, C.-M.; Choi, J.-d.-R.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-L.; Guo, W.-Q.; Zheng, H.-S.; Luo, H.-C.; Feng, X.-C.; Yin, R.-L.; Ren, N.-Q. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: The mechanism and microbial community analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Wang, K.; Shen, D. Acidogenic fermentation of the main substrates of food waste to produce volatile fatty acids. Int. J. Hydrogen Energy 2016, 41, 21713–21720. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Zou, D.; Yuan, H.; Zhu, B.; Li, X.; Pang, Y. Influence of Temperature on Hydrolysis Acidification of Food Waste. Procedia Environ. Sci. 2012, 16, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Voelklein, M.A.; Jacob, A.; O’ Shea, R.; Murphy, J.D. Assessment of increasing loading rate on two-stage digestion of food waste. Bioresour. Technol. 2016, 202, 172–180. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev. Environ. Sci. Bio/Technol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Wei, D.; Liu, X.; Yang, S.-T. Butyric acid production from sugarcane bagasse hydrolysate by Clostridium tyrobutyricum immobilized in a fibrous-bed bioreactor. Bioresour. Technol. 2013, 129, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, Q.; Li, H.; Zhang, Y.; Deng, Z.; Liu, J.; Du, X. Effect of fermentation type regulation using alkaline addition on two-phase anaerobic digestion of food waste at different organic load rates. Renew. Energy 2020, 154, 385–393. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zhao, S.; Wang, D.; Zheng, X.; Luo, J. Lactic acid accumulation from sludge and food waste to improve the yield of propionic acid-enriched VFA. Biochem. Eng. J. 2014, 84, 28–35. [Google Scholar] [CrossRef]
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic digestion of food waste in a thermophilic leach bed reactor: Effect of pH and leachate recirculation rate on hydrolysis and volatile fatty acid production. Bioresour. Technol. 2017, 245, 1–9. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Karabiyikli, S. Importance of acetic acid bacteria in food industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Niu, D.; Zhao, Y. Acetic acid production from food wastes using yeast and acetic acid bacteria micro-aerobic fermentation. Bioprocess Biosyst. Eng. 2015, 38, 863–869. [Google Scholar] [CrossRef]
- Arras, W.; Hussain, A.; Hausler, R.; Guiot, S.R. Mesophilic, thermophilic and hyperthermophilic acidogenic fermentation of food waste in batch: Effect of inoculum source. Waste Manag. 2019, 87, 279–287. [Google Scholar] [CrossRef]
- Xu, S.; Selvam, A.; Wong, J.W.C. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014, 34, 363–369. [Google Scholar] [CrossRef]
- Luo, J.; Feng, L.; Zhang, W.; Li, X.; Chen, H.; Wang, D.; Chen, Y. Improved production of short-chain fatty acids from waste activated sludge driven by carbohydrate addition in continuous-flow reactors: Influence of SRT and temperature. Appl. Energy 2014, 113, 51–58. [Google Scholar] [CrossRef]
- Niphadkar, S.; Bagade, P.; Ahmed, S. Bioethanol production: insight into past, present and future perspectives. Biofuels 2018, 9, 229–238. [Google Scholar] [CrossRef]
- Soccol, C.R.; Faraco, V.; Karp, S.G.; Vandenberghe, L.P.S.; Thomaz-Soccol, V.; Woiciechowski, A.L.; Pandey, A. Chapter 14—Lignocellulosic Bioethanol: Current Status and Future Perspectives. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 331–354. [Google Scholar]
- Zheng, M.; Zheng, M.; Wu, Y.; Ma, H.; Wang, K. Effect of pH on types of acidogenic fermentation of fruit and vegetable wastes. Biotechnol. Bioprocess Eng. 2015, 20, 298–303. [Google Scholar] [CrossRef]
- Ren, N.; Wang, B.; Huang, J.-C. Ethanol-type fermentation from carbohydrate in high rate acidogenic reactor. Biotechnol. Bioeng. 1997, 54, 428–433. [Google Scholar] [CrossRef]
- Yan, S.; Li, J.; Chen, X.; Wu, J.; Wang, P.; Ye, J.; Yao, J. Enzymatical hydrolysis of food waste and ethanol production from the hydrolysate. Renew. Energy 2011, 36, 1259–1265. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Koike, Y.; Liu, K.; An, M.-Z.; Morimura, S.; Wu, X.-L.; Kida, K. Ethanol production from kitchen waste using the flocculating yeast Saccharomyces cerevisiae strain KF-7. Biomass Bioenergy 2008, 32, 1037–1045. [Google Scholar] [CrossRef]
- Ajit, A.; Sulaiman, A.Z.; Chisti, Y. Production of bioethanol by Zymomonas mobilis in high-gravity extractive fermentations. Food Bioprod. Process. 2017, 102, 123–135. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A review of recent advances in high gravity ethanol fermentation. Renew. Energy 2019, 133, 1366–1379. [Google Scholar] [CrossRef]
- Fan, S.; Xiao, Z.; Tang, X.; Chen, C.; Zhang, Y.; Deng, Q.; Yao, P.; Li, W. Inhibition effect of secondary metabolites accumulated in a pervaporation membrane bioreactor on ethanol fermentation of Saccharomyces cerevisiae. Bioresour. Technol. 2014, 162, 8–13. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, J.; Bao, J. Inhibitor analysis and adaptive evolution of Saccharomyces cerevisiae for simultaneous saccharification and ethanol fermentation from industrial waste corncob residues. Bioresour. Technol. 2014, 157, 6–13. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: a biorefinery perspective. Int. J. Food Sci. Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bonk, F.; Bastidas-Oyanedel, J.-R.; Yousef, A.F.; Schmidt, J.E. Exploring the selective lactic acid production from food waste in uncontrolled pH mixed culture fermentations using different reactor configurations. Bioresour. Technol. 2017, 238, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y. Dynamic membrane-assisted fermentation of food wastes for enhancing lactic acid production. Bioresour. Technol. 2017, 234, 40–47. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Environmental stress responses in Lactobacillus: A review. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef]
- Kim, M.-S.; Na, J.-G.; Lee, M.-K.; Ryu, H.; Chang, Y.-K.; Triolo, J.M.; Yun, Y.-M.; Kim, D.-H. More value from food waste: Lactic acid and biogas recovery. Water Res. 2016, 96, 208–216. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Tufvesson, P.; Ekman, A.; Sardari, R.R.R.; Engdahl, K.; Tufvesson, L. Economic and environmental assessment of propionic acid production by fermentation using different renewable raw materials. Bioresour. Technol. 2013, 149, 556–564. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zheng, X.; Wang, D. Enhancement of propionic acid fraction in volatile fatty acids produced from sludge fermentation by the use of food waste and Propionibacterium acidipropionici. Water Res. 2013, 47, 615–622. [Google Scholar] [CrossRef]
- Ren, N.Q.; Chua, H.; Chan, S.Y.; Tsang, Y.F.; Wang, Y.J.; Sin, N. Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour. Technol. 2007, 98, 1774–1780. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Li, F.T. Avoiding propionic acid accumulation in the anaerobic process for biohydrogen production. Biomass Bioenergy 2006, 30, 177–182. [Google Scholar] [CrossRef]
- Mascal, M. Chemicals from biobutanol: technologies and markets. Biofuels Bioprod. Biorefin. 2012, 6, 483–493. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Su, G.; He, J. Enhanced direct fermentation from food waste to butanol and hydrogen by an amylolytic Clostridium. Renew. Energy 2020, 153, 7. [Google Scholar] [CrossRef]
- Huang, H.; Singh, V.; Qureshi, N. Butanol production from food waste: a novel process for producing sustainable energy and reducing environmental pollution. Biotechnol. Biofuels 2015, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Chen, J.-N.; He, A.-Y.; Wu, H.; Kong, X.-P.; Liu, J.-L.; Yin, C.-Y.; Chen, W.-F.; Chen, P. Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochem. 2014, 49, 1238–1244. [Google Scholar] [CrossRef]
- Li, S.-Y.; Srivastava, R.; Suib, S.L.; Li, Y.; Parnas, R.S. Performance of batch, fed-batch, and continuous A–B–E fermentation with pH-control. Bioresour. Technol. 2011, 102, 4241–4250. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, X.; Ma, H. Glucoamylase production from food waste by Aspergillus niger under submerged fermentation. Process Biochem. 2008, 43, 280–286. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.-Z.; Lam, K.F.; Lin, C.S.K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Wang, M.; Wang, W.; Deng, L.; Nie, K.; Yue, X.; Wang, F.; Tan, T. Food Waste Fermentation to Fumaric Acid by Rhizopus arrhizus RH7-13. Appl. Biochem. Biotechnol. 2016, 180, 1524–1533. [Google Scholar] [CrossRef]
- Connaughton, S.; Collins, G.; O’Flaherty, V. Psychrophilic and mesophilic anaerobic digestion of brewery effluent: A comparative study. Water Res. 2006, 40, 2503–2510. [Google Scholar] [CrossRef]
- Bouallagui, H.; Haouari, O.; Touhami, Y.; Ben Cheikh, R.; Marouani, L.; Hamdi, M. Effect of temperature on the performance of an anaerobic tubular reactor treating fruit and vegetable waste. Process Biochem. 2004, 39, 2143–2148. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, K.; Sánchez, E.; El-Halwagi, M.; Kafarov, V. Exergy analysis and process integration of bioethanol production from acid pre-treated biomass: Comparison of SHF, SSF and SSCF pathways. Chem. Eng. J. 2011, 176–177, 195–201. [Google Scholar] [CrossRef]
- Khosravanipour Mostafazadeh, A.; Drogui, P.; Brar, S.K.; Tyagi, R.D.; Bihan, Y.L.; Buelna, G. Microbial electrosynthesis of solvents and alcoholic biofuels from nutrient waste: A review. J. Environ. Chem. Eng. 2017, 5, 940–954. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W.C. Hydrolysis–acidogenesis of food waste in solid–liquid-separating continuous stirred tank reactor (SLS-CSTR) for volatile organic acid production. Bioresour. Technol. 2016, 200, 366–373. [Google Scholar] [CrossRef]
- Yun, J.; Cho, K.-S. Effects of organic loading rate on hydrogen and volatile fatty acid production and microbial community during acidogenic hydrogenesis in a continuous stirred tank reactor using molasses wastewater. J. Appl. Microbiol. 2016, 121, 1627–1636. [Google Scholar] [CrossRef]
- Browne, J.D.; Allen, E.; Murphy, J.D. Improving hydrolysis of food waste in a leach bed reactor. Waste Manag. 2013, 33, 2470–2477. [Google Scholar] [CrossRef]
- Xu, S.Y.; Karthikeyan, O.P.; Selvam, A.; Wong, J.W.C. Microbial community distribution and extracellular enzyme activities in leach bed reactor treating food waste: Effect of different leachate recirculation practices. Bioresour. Technol. 2014, 168, 41–48. [Google Scholar] [CrossRef]
- Xu, S.Y.; Lam, H.P.; Karthikeyan, O.P.; Wong, J.W.C. Optimization of food waste hydrolysis in leach bed coupled with methanogenic reactor: Effect of pH and bulking agent. Bioresour. Technol. 2011, 102, 3702–3708. [Google Scholar] [CrossRef]
- Xiong, Z.; Hussain, A.; Lee, J.; Lee, H.-S. Food waste fermentation in a leach bed reactor: Reactor performance, and microbial ecology and dynamics. Bioresour. Technol. 2019, 274, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Application of rumen microbes to enhance food waste hydrolysis in acidogenic leach-bed reactors. Bioresour. Technol. 2014, 168, 64–71. [Google Scholar] [CrossRef]
- Walker, M.; Banks, C.J.; Heaven, S. Two-stage anaerobic digestion of biodegradable municipal solid waste using a rotating drum mesh filter bioreactor and anaerobic filter. Bioresour. Technol. 2009, 100, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; Pepé Sciarria, T.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-Fermentation—Merging Electrochemistry with Fermentation in Industrial Applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef]
- Paiano, P.; Menini, M.; Zeppilli, M.; Majone, M.; Villano, M. Electro-fermentation and redox mediators enhance glucose conversion into butyric acid with mixed microbial cultures. Bioelectrochemistry 2019, 130, 107333. [Google Scholar] [CrossRef]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How To Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Rago, L.; Pant, D.; Schievano, A. Chapter 14-Electro-Fermentation—Microbial Electrochemistry as New Frontier in Biomass Refineries and Industrial Fermentations. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Hosseini, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 265–287. [Google Scholar]
- Mathew, A.S.; Wang, J.; Luo, J.; Yau, S.-T. Enhanced ethanol production via electrostatically accelerated fermentation of glucose using Saccharomyces cerevisiae. Sci. Rep. 2015, 5, 15713. [Google Scholar] [CrossRef] [Green Version]
- Xue, G.; Lai, S.; Li, X.; Zhang, W.; You, J.; Chen, H.; Qian, Y.; Gao, P.; Liu, Z.; Liu, Y. Efficient bioconversion of organic wastes to high optical activity of l-lactic acid stimulated by cathode in mixed microbial consortium. Water Res. 2018, 131, 1–10. [Google Scholar] [CrossRef]
- Luo, J.; Feng, L.; Chen, Y.; Li, X.; Chen, H.; Xiao, N.; Wang, D. Stimulating short-chain fatty acids production from waste activated sludge by nano zero-valent iron. J. Biotechnol. 2014, 187, 98–105. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Q.; Wu, S.; Luo, J.; Wu, Y.; Zhang, L.; Feng, Q.; Fang, F.; Xue, Z. Enhancing the anaerobic bioconversion of complex organics in food wastes for volatile fatty acids production by zero-valent iron and persulfate stimulation. Sci. Total Environ. 2019, 669, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Luo, Q.; Wang, L.; Liu, S.; Peng, Y.; Wang, H.; Shen, L.; Li, Q.; Wang, Y. Maghemite (γ-Fe2O3) nanoparticles enhance dissimilatory ferrihydrite reduction by Geobacter sulfurreducens: Impacts on iron mineralogical change and bacterial interactions. J. Environ. Sci. 2019, 78, 193–203. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, Y.; Quan, X.; Zhao, Z. Comparing the mechanisms of ZVI and Fe3O4 for promoting waste-activated sludge digestion. Water Res. 2018, 144, 126–133. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Chang, J.; Quan, X.; Li, Q. Biological sulfate reduction in the acidogenic phase of anaerobic digestion under dissimilatory Fe (III)—Reducing conditions. Water Res. 2013, 47, 2033–2040. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Duan, X.; Feng, L.; Yan, Y.; Wang, F.; Zhang, X.; Zhang, Z.; Zhou, Q. Activated carbon promotes short-chain fatty acids production from algae during anaerobic fermentation. Sci. Total Environ. 2019, 658, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Kumar, A.; Zhang, H. Enhanced ethanol production by Clostridium ragsdalei from syngas by incorporating biochar in the fermentation medium. Bioresour. Technol. 2018, 247, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chen, Y.; Yan, Y.; Feng, L.; Chen, Y.; Zhou, Q. New method for algae comprehensive utilization: Algae-derived biochar enhances algae anaerobic fermentation for short-chain fatty acids production. Bioresour. Technol. 2019, 289, 121637. [Google Scholar] [CrossRef]
- Li, Q.Z.; Jiang, X.L.; Feng, X.J.; Wang, J.M.; Sun, C.; Zhang, H.B.; Xian, M.; Liu, H.Z. Recovery Processes of Organic Acids from Fermentation Broths in the Biomass-Based Industry. J. Microbiol. Biotechnol. 2016, 26, 1–8. [Google Scholar] [CrossRef]
- Bak, C.; Yun, Y.-M.; Kim, J.-H.; Kang, S. Electrodialytic separation of volatile fatty acids from hydrogen fermented food wastes. Int. J. Hydrogen Energy 2019, 44, 3356–3362. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, J.; Bryjak, M.; Kujawski, W. ABE fermentation products recovery methods—A review. Renew. Sustain. Energy Rev. 2015, 48, 648–661. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, K.; Harwardt, A.; Bronneberg, R.; Marquardt, W. Separation of butanol from acetone–butanol–ethanol fermentation by a hybrid extraction–distillation process. Comput. Chem. Eng. 2011, 35, 949–963. [Google Scholar] [CrossRef]
- Haelssig, J.B.; Tremblay, A.Y.; Thibault, J. A new hybrid membrane separation process for enhanced ethanol recovery: Process description and numerical studies. Chem. Eng. Sci. 2012, 68, 492–505. [Google Scholar] [CrossRef]
- Yang, S.-T.; Huang, H.; Tay, A.; Qin, W.; De Guzman, L.; Nicolas, E.C.S. Chapter 16-Extractive Fermentation for the Production of Carboxylic Acids. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 421–446. [Google Scholar]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Separation techniques in butanol production: Challenges and developments. Biomass Bioenergy 2014, 60, 222–246. [Google Scholar] [CrossRef]
- Hu, Y.; Kwan, T.H.; Daoud, W.A.; Lin, C.S.K. Continuous ultrasonic-mediated solvent extraction of lactic acid from fermentation broths. J. Clean. Prod. 2017, 145, 142–150. [Google Scholar] [CrossRef]
- Woo, H.C.; Kim, Y.H. Eco-efficient recovery of bio-based volatile C2–6 fatty acids. Biotechnol. Biofuels 2019, 12, 92. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, A.; Bonk, F.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Tang, I.C.; Zhao, J.; Bai, F.; Yang, S.-T. Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Bioresour. Technol. 2016, 219, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Thuy, N.T.H.; Boontawan, A. Production of very-high purity succinic acid from fermentation broth using microfiltration and nanofiltration-assisted crystallization. J. Membr. Sci. 2017, 524, 470–481. [Google Scholar] [CrossRef]
- Lewandowicz, G.; Białas, W.; Marczewski, B.; Szymanowska, D. Application of membrane distillation for ethanol recovery during fuel ethanol production. J. Membr. Sci. 2011, 375, 212–219. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, H.; Wang, Q.; Zhao, F.; Xiao, Z. Pretreatment technology for suspended solids and oil removal in an ethanol fermentation broth from food waste separated by pervaporation process. Desalination 2012, 293, 112–117. [Google Scholar] [CrossRef]




| Food Waste Source | Total Solid (TS, %) | Volatile Solid (VS) in TS (%) | Carbohydrate | Protein | Lipid | Carbon to Nitrogen Ratio | References |
|---|---|---|---|---|---|---|---|
| San Francisco, USA | 30.9 | 85.3 | 14.5 | [7] | |||
| Grand Narbonne, France | 21.0 | 90.3 | 618 g/kg TS | 187 g/kg TS | 121 g/kg TS | 16.1 | [8] |
| Korea | 12.4 | 89.3 | 9.2 | [9] | |||
| A restaurant in Myongji University, Korea | 18.1 | 94.0 | 111.7 g/L | 32.9 g/L | 23.3 g/L | 13.2 | [10] |
| Japan | 16.5 | 94.0 | 13.2 | [11] | |||
| A dining hall of National Institute for Environmental Studies, Japan | 33.8 | 92.0 | 66 g/L (55–69% in VS) | 45 g/L (37–44% in VS) | [12] | ||
| Beijing University of Chemical Technology, China | 18.5 | 92.0 | 22.8% in TS | 21.1 | [13] | ||
| A dining hall of Tongji University, China | 23.2 | 88.4 | 135.6 g/L | 42.27 g/L | 13.61 g/L | 32.0 | [14] |
| Residential homes in Davis, CA | 19.2 | 92.7 | 34.7% in TS | 17.1 | [15] | ||
| A canteen in University College Cork, Ireland | 29.4 | 95.3 | 59% in TS | 18.1% in TS | 18% in TS | 14.2 | [16] |
| Inoculum | pH | Total Solid (%) a | Volatile Solid in Total Solid (%) | References |
| Anaerobic sludge | 6.8 | 12 | 70 b | [30] |
| Aerobic sludge | 6.9 | 6.3 | 72 b | |
| Anaerobic sludge | 7.6 ± 0.2 | 1.8 ± 0.34 | 55.56 b | [46] |
| Anaerobic sludge | 7.3 | 2.1 ± 0.5 | 68.5 ± 15.3 | [42] |
| Fresh food waste from a cafeteria | 4.3 | 4.3 ± 0.3 | 96.4 ± 7.6 | |
| Food waste collected from a cafeteria | 5 | 11.18 | 94.9 | [41] |
| Digested dairy manure | \ | 2.8 | 59.1 | [44] |
| Dairy manure | 7.6 ± 0.1 | 22.3 ± 0.3 | 68.6 ± 0.3 | [47] |
| Cattle dung | 6.8 | 5.37 | 53.26 | [48] |
| Products | Inoculum | pH | Temperature | OLR g/(L·d) | ORP |
|---|---|---|---|---|---|
| mixed VFAs | Mixed inoculation | 5.5–11.0 | Mesophilic | Moderate | - |
| Acetic acid | Mixed inoculation | 8.0–10.0 | Mesophilic | Low or Moderate | strict anaerobic |
| butyric acid | Mixed inoculation | 5.0–6.5 | Thermophilic | Moderate | ˂−250 mV |
| Ethanol | Mixed inoculation | 4.0–4.5 | Mesophilic | Moderate | ~0 mV |
| Ethanol | Yeast | 4.5–5.5 | 20–35 °C | Moderate or High | >−150 mV |
| Ethanol | Zymomonas mobilis | 5.0–5.5 | 30–35 °C | - | - |
| Lactic acid | lactobacillus | 5.0–7.0 | 20–45 °C | Moderate | −400–100 mV |
| Propionic acid | Mixed inoculation | 5.0–5.5 | Mesophilic | High | >−240 mV |
| Butanol | Clostridium | 4.5–6.0 | Mesophilic | - | strict anaerobic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies 2020, 13, 5638. https://doi.org/10.3390/en13215638
Wang Q, Li H, Feng K, Liu J. Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies. 2020; 13(21):5638. https://doi.org/10.3390/en13215638
Chicago/Turabian StyleWang, Qiao, Huan Li, Kai Feng, and Jianguo Liu. 2020. "Oriented Fermentation of Food Waste towards High-Value Products: A Review" Energies 13, no. 21: 5638. https://doi.org/10.3390/en13215638
APA StyleWang, Q., Li, H., Feng, K., & Liu, J. (2020). Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies, 13(21), 5638. https://doi.org/10.3390/en13215638