Synthesis and Application of Heterogeneous Catalysts Based on Heteropolyacids for 5-Hydroxymethylfurfural Production from Glucose
Abstract
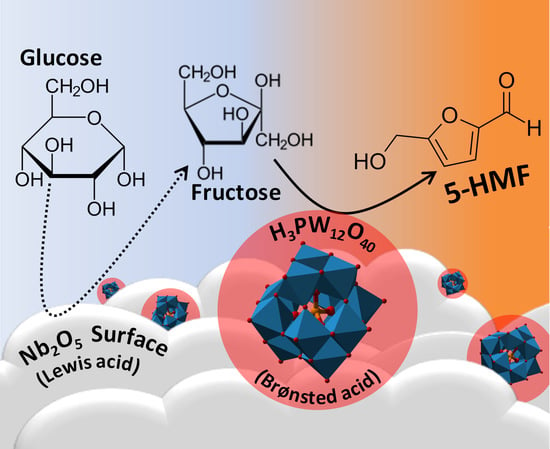
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. HMF Production
2.3. Analytical Methods
3. Results and Discussion
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Reaction Conditions Evaluation
3.4. Catalyst Recycling Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I. (Ed.) Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier Inc.: Waltham, MA, USA, 2016; p. 674. ISBN 9780128023235. [Google Scholar]
- Werpy, T.; Petersen, G.; National Renewable Energy Laboratory (NREL). Top Value Added Chemicals from Biomass. Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. 2004. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 3 September 2019).
- Mukherjee, A.; Dumont, M.J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Jong, E.; Dam, M.A.; Sipos, L.; Gruter, J.M. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters. In Biobased Monomers, Polymers, and Materials; Smith, P.B., Gross, R.A., Eds.; American Chemical Society: Midland, MI, USA, 2012; Volume 1105, pp. 1–13. [Google Scholar] [CrossRef]
- Sweygers, N.; Alewaters, N.; Dewil, R.; Appels, L. Microwave effects in the dilute acid hydrolysis of cellulose to 5-hydroxymethylfurfural. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Candu, N.; Fergani, M.E.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Lopes, M.; Dussan, K.; Leahy, J.J.; Da Silva, V.T. Conversion of d-glucose to 5-hydroxymethylfurfural using Al2O3-promoted sulphated tin oxide as catalyst. Catal. Today 2017, 279, 233–243. [Google Scholar] [CrossRef]
- Rusanen, A.; Lahti, R.; Lappalainen, K.; Kärkkäinen, J.; Hu, T.; Romar, H.; Lassi, U. Catalytic conversion of glucose to 5-hydroxymethylfurfural over biomass based activated carbon catalyst. Catal. Today 2019. [Google Scholar] [CrossRef]
- Shahangi, F.; Chermahini, A.N.; Saraji, M. Dehydration of fructose and glucose to 5-hydroxymethylfurfural over Al-KCC-1 silica. J. Energy Chem. 2018, 27, 769–780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Li, W.; Xin, H.; Jin, L.; Liu, Q. Production of HMF from glucose using an Al3+-promoted acidic phenol-formaldehyde resin catalyst. Catal. Commun. 2019, 124, 56–61. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Appl. Catal. B Environ. 2019, 253, 1–10. [Google Scholar] [CrossRef]
- Daorattanachai, P.; Khemthong, P.; Viriya-empikul, N.; Laosiripojana, N.; Faungnawakij, K. Effect of calcination temperature on catalytic performance of alkaline earth phosphates in hydrolysis/dehydration of glucose and cellulose. Chem. Eng. 2015, 278, 92–98. [Google Scholar] [CrossRef]
- Gomes, F.N.D.C.; Pereira, L.R.; Riberiro, N.F.P.; Souza, M.M.V.M. Production of 5-hydroxymethylfurfural (HMF) via fructose dehydration: Effect of solvent and salting-out. Braz. J. Chem. Eng. 2015, 32, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Guan, J.; Peng, J.; Guang-Zhou, Z.; Ji-Hong, J. Green hydrolysis of corncob cellulose into 5-hydroxymethylfurfural using hydrophobic imidazole ionic liquids with a recyclable, magnetic metalloporphyrin catalyst. Chem. Eng. J. 2017, 330, 109–119. [Google Scholar] [CrossRef]
- Yan, D.; Xin, J.; Shi, C.; Lu, X.; Ni, L.; Wang, G.; Zhang, S. Base-free conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid in ionic liquids. Chem. Eng. J. 2017, 323, 473–482. [Google Scholar] [CrossRef]
- Boisen, A.; Christensen, T.B.; Fu, W.; Gorbanev, Y.Y.; Hansen, T.S.; Jensen, J.S.; Klitgaad, S.K.; Pedersen, S.; Riisager, A.; Stahlberg, T.; et al. Process integration for the conversion of glucose to 2,5-furandicarboxilic acid. Chem. Eng. Res. Des. 2009, 87, 1318–1327. [Google Scholar] [CrossRef]
- Kale, S.S.; Armbruster, U.; Eckelt, R.; Bentrup, U.; Umbarkar, S.B.; Dongare, M.K.; Martin, A. Understanding the role of Keggin type heteropolyacid catalysts for glycerol acetylation using toluene as an entrainer. Appl. Catal. A Gen. 2016, 527, 9–18. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Méndez, F.J.; Llanos, A.; Echeverría, M.; Jáuregui, R.; Villasana, Y.; Díaz, Y.; Liendo-Polanco, G.; Ramos-García, M.A.; Zoltan, T.; Brito, J.L. Mesoporous catalysts based on Keggin-type heteropolyacids supported on MCM-41 and their application in thiphene hydrodesulfurization. Fuel 2013, 110, 249–258. [Google Scholar] [CrossRef]
- Marcí, G.; García-Lopez, E.; Vaiano, V.; Sarno, G.; Sannino, D.; Palmisano, L. Keggin heteropolyacid supported on TiO2 used in gas-solid (photo)catalytic propene hydration and in liquid-solid photocatalytic glycerol dehydration. Catal. Today 2017, 281, 60–70. [Google Scholar] [CrossRef]
- Han, X.; Yan, W.; Chen, K.; Hung, C.T.; Liu, L.L.; Wu, P.H.; Huang, S.J.; Liu, S.B. Heteropolyacid-based ionic liquids as effective catalysts for the synthesis of benzaldehyde glycol acetal. Appl. Catal. A Gen. 2014, 485, 149–156. [Google Scholar] [CrossRef]
- Pinto, T.; Dufaud, V.; Lefebvre, F. Isomerization of n-hexane on heteropolyacids supported on SBA-15. 1. Monofunctional impregnated catalysts. Appl. Catal. A Gen. 2014, 483, 103–109. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Lin, L.; Liu, S. 12-Tungstophosphoric acid/boric acid as synergetic catalysts for the conversion of glucose into 5-hydroxymethylfurfural in ionic liquid. Biomass Bioenergy 2012, 47, 289–294. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Wang, Y.; Cui, H.; Song, F.; Sun, X.; Zhang, L. Conversion of glucose into 5-hydroxymethylfurfural catalyzed by acid–base bifunctional heteropolyacid-based ionic hybrids. Green Chem. 2018, 20, 1551–1559. [Google Scholar] [CrossRef]
- Fan, C.; Guan, H.; Zhang, H.; Wang, J.; Wang, S.; Wang, X. Conversion of fructose and glucose into 5-hydroxymethylfurfural catalyzed by a solid heteropolyacid salt. Biomass Bioenergy 2011, 35, 2659–2665. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Tao, Y.; Sun, W.; Wang, W. Preparation of 5-hydroxymethylfurfural from glucose catalyzed by silicasupported phosphotungstic acid heterogeneous catalyst. Fuel 2018, 226, 417–422. [Google Scholar] [CrossRef]
- Martín, C.; Solana, G.; Malet, P.; Rives, V. Nb2O5-supported WO3: A comparative study with WO3/Al2O3. Catal. Today 2003, 78, 365–376. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Sustainable heterogeneous acid catalysis by heteropoly acids. J. Mol. Catal. A Chem. 2007, 262, 86–92. [Google Scholar] [CrossRef]
- Alsalme, A.M.; Wiper, P.V.; Khimyak, Y.Z.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Solid acid catalysts based on H3PW12O40 heteropoly acid: Acid and catalytic properties at a gas–solid interface. J. Catal. 2010, 276, 181–189. [Google Scholar] [CrossRef]
- Keggin, J.F. Structure and formula of 12-phosphotungstic acid. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1934, 144, 75–100. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Huang, Y.; Zhou, Y.; Liu, H.; Cai, Y.; Lu, S.; Yao, Y. Homogeneously dispersed HPW/graphene for high efficient catalytic oxidative desulfurization prepared by electrochemical deposition. Appl. Surf. Sci. 2019, 484, 917–924. [Google Scholar] [CrossRef]
- Caliman, E.; Dias, J.A.; Dias, S.C.L.; Garcia, F.A.C.; Macedo, J.L.D.; Almeida, L.S. Preparation and characterization of H3PW12O40 supported on niobia. Microporous Mesoporous Mater. 2010, 132, 103–111. [Google Scholar] [CrossRef]
- Conceição, L.R.V.D.; Carneiro, L.M.; Giordani, D.S.; Castro, H.F.D. Synthesis of biodiesel from macaw palm oil using mesoporous solid catalyst comprising 12-molybdophosphoric acid and niobia. Renew. Energy 2017, 113, 119–128. [Google Scholar] [CrossRef]
- Peela, N.R.; Yedla, S.K.; Velaga, B.; Kumar, A.; Golder, A.K. Choline chloride functionalized zeolites for the conversion of biomass derivatives to 5-hydroxymethykfurfural. Appl. Catal. A Gen. 2019, 580, 59–70. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Effect of Lewis and Brønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl. Catal. A Gen. 2018, 555, 75–87. [Google Scholar] [CrossRef]
- García-Sancho, C.; Fúnez-Núñez, I.; Moreno-Tost, T.; Santamaría-González, J.; Pérez-Inestrosa, E.; Fierro, J.L.G.; Maireles-Torres, P. Beneficial effects of calcium chloride on glucose dehydration to5-hydroxymethylfurfural in the presence of alumina as catalyst. Appl. Catal. B Environ. 2017, 206, 617–625. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Su, D. 5-Hydroxymethylfurfural: A key intermediate for efficient biomass conversion. J. Energy Chem. 2015, 24, 548–551. [Google Scholar] [CrossRef]
- Teimouri, A.; Mazaheri, M.; Chermahini, A.N.; Salavati, H.; Momenbeik, F.; Fazel-Najafabadi, M. Catalytic conversion of glucose to 5-hydroxymethylfurfural (HMF) using nano-POM/nano-ZrO2/nano-γ-Al2O3. J. Taiwan Inst. Chem. Eng. 2015, 49, 40–50. [Google Scholar] [CrossRef]
- Shen, Y.; Kang, Y.; Sun, J.; Wang, C.; Wang, B.; Xu, F.; Sun, R. Efficient production of 5-hydroxymethylfurfural from hexoses using solid acid SO4-2/In2O3-ATP in a biphasic system. Chin. J. Catal. 2016, 37, 1362–1368. [Google Scholar] [CrossRef]
- Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P. Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 303, 22–30. [Google Scholar] [CrossRef]






| Catalyst | Experimental Conditions (Factors and Interactions) | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A Temp. (°C) | B Support | AB | C Active phase | AC | BC | ABC | Y HMF 1(%) | XGlu 2(%) | |
| 1 | 300 | Nb | 1 | HPMo | 1 | 1 | 1 | 3.0 | 92.3 |
| 2.8 | 93.6 | ||||||||
| 2 | 300 | Nb | 1 | HPW | 2 | 2 | 2 | 9.5 | 75.0 |
| 9.4 | 75.7 | ||||||||
| 3 | 300 | Al | 2 | HPMo | 1 | 2 | 2 | 4.4 | 90.3 |
| 3.7 | 85.3 | ||||||||
| 4 | 300 | Al | 2 | HPW | 2 | 1 | 1 | 0.8 | 69.8 |
| 0.8 | 67.2 | ||||||||
| 5 | 500 | Nb | 2 | HPMo | 2 | 1 | 2 | 6.9 | 91.8 |
| 7.0 | 90.7 | ||||||||
| 6 | 500 | Nb | 2 | HPW | 1 | 2 | 1 | 0.7 | 65.3 |
| 0.8 | 66.1 | ||||||||
| 7 | 500 | Al | 1 | HPMo | 2 | 2 | 1 | 7.3 | 91.0 |
| 7.7 | 87.6 | ||||||||
| 8 | 500 | Al | 1 | HPW | 1 | 1 | 2 | 0.7 | 70.6 |
| 0.7 | 68.9 | ||||||||
| Source of Variation | Response Variables | |
|---|---|---|
| p-Value for Y HMF | p-Value for XGlu | |
| (A) Temperature | 0.0155 * | 0.0413 * |
| (B) Support | <0.0001 * | 0.0237 * |
| (D) Active phase | <0.0001 * | 0.0001 * |
| AB | <0.0001 * | 0.0041 * |
| AD | <0.0001 * | 0.0472 * |
| BD | <0.0001 * | 0.2526 |
| ABD | <0.0001 * | 0.0626 |
| R² | 0.9978 | 0.9862 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Surface Acidity (μmol H+/ m2) |
|---|---|---|---|
| Nb2O5 | 130.91 | 0.14 | 0.31 |
| HPW/Nb2O5-300 °C | 36.08 | 0.031 | 106.98 |
| Experimental Conditions (Factors and Interactions) | Response Variables | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | A Temp. (°C) | B Acetone: Water (v/v) | AB | C CGlu (g/L) | AC | BC | CE | D Cat. (%w/v) | AD | BD | CE | CD | BE | AE | E 1 | YHMF 2 (%) | XGlu 3 (%) |
| 1 | 160 | 1:1 | 1 | 50 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15.4 | 71.3 |
| 2 | 160 | 1:1 | 1 | 50 | 1 | 1 | 1 | 5 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 40.8 | 93.3 |
| 3 | 160 | 1:1 | 1 | 100 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 8.8 | 63.7 |
| 4 | 160 | 1:1 | 1 | 100 | 2 | 2 | 2 | 5 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 30.1 | 86.1 |
| 5 | 160 | 3:1 | 2 | 50 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 17.5 | 80.9 |
| 6 | 160 | 3:1 | 2 | 50 | 1 | 2 | 2 | 5 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 40.5 | 93.3 |
| 7 | 160 | 3:1 | 2 | 100 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 7.6 | 63.9 |
| 8 | 160 | 3:1 | 2 | 100 | 2 | 1 | 1 | 5 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 32.6 | 88.0 |
| 9 | 200 | 1:1 | 2 | 50 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 11.5 | 97.4 |
| 10 | 200 | 1:1 | 2 | 50 | 2 | 1 | 2 | 5 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 14.2 | 97.9 |
| 11 | 200 | 1:1 | 2 | 100 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 8.5 | 96.0 |
| 12 | 200 | 1:1 | 2 | 100 | 1 | 2 | 1 | 5 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 9.8 | 98.4 |
| 13 | 200 | 3:1 | 1 | 50 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 20.8 | 95.9 |
| 14 | 200 | 3:1 | 1 | 50 | 2 | 2 | 1 | 5 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 14.0 | 96.4 |
| 15 | 200 | 3:1 | 1 | 100 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 18.8 | 73.0 |
| 16 | 200 | 3:1 | 1 | 100 | 1 | 1 | 2 | 5 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 17.0 | 85.5 |
| Source of Variation | Response Variables | |
|---|---|---|
| p-Value for Y HMF | p-Value for XGlu | |
| (A) Treaction | 0.000 * | 0.002 * |
| (B) Acetone:Water | 0.027 * | 0.168 |
| (C) Cglucose | 0.007 * | 0.008 * |
| (D) Ccatalyst | 0.000 * | 0.002 * |
| AB | 0.058 | 0.031 * |
| AC | 0.030 * | 0.889 |
| AD | 0.000 * | 0.012 * |
| BC | 0.448 | 0.063 |
| BD | 0.290 | 0.902 |
| CD | 0.874 | 0.185 |
| R² | 0.984 | 0.960 |
| Catalyst | Solvent | Cglu | T (°C) | T (min) | YHMF (%) | Ref. |
|---|---|---|---|---|---|---|
| Nano-POM a/nano-ZrO2/nano-γ-Al2O3 | Water/acetone | 4 g/L | 190 | 240 | 34.6 | [38] |
| Sn/γ-Al2O3 | Water/DMSO (1:4 v/v) | 4 wt.% | 150 | 60 | 27.5 | [35] |
| GO b-Fe2O3 | DMSO | 180 g/L | 140 | 240 | 16 | [37] |
| Al2O3 treated with 0.05 M NaOH | EMIMCl | 10 wt.% | 140 | 120 | 36 | [11] |
| ACBL2 c | Water | 11.25 g/L | 160 | 480 | 15 | [8] |
| TSA350 d | Water | 36 g/L | 120 | 360 | 19 | [7] |
| Acid γ-Al2O3 | Aq. solution of CaCl2/ MIBK (1:2.3 v/v) | 30 g/L | 175 | 15 | 23 | [36] |
| Al-KCC-1 e | DMSO | 125 g/L | 170 | 120 | 39 | [9] |
| S-TsC f | Water/γ-valerolactone (GVL) (0.3:4.7 v/v) | 20 g/L | 160 | 30 | 27.8 | [26] |
| HMOR_20 g | Aq. solution of NaCl/ MIBK (2.6:4 w/w) | 3.33 wt.% | 180 | 60 | 27 | [34] |
| SO42-/ In2O3-ATP h | Water/GVL (1:9 v/v) | 2 wt.% | 180 | 60 | 40.2 | [39] |
| Al-SPFR i | Water/GVL (1:10 v/v) | 12.1 g/L | 180 | 50 | 41.5 | [10] |
| H-ZSM-5 zeolite | Aq. solution of NaCl/ MIBK (1.5:3.5 v/v) | 30 g/L | 195 | 30 | 42 | [40] |
| HPW/Nb2O5-300 °C | Water/acetone (1:1 v/v) | 50 g/L | 160 | 30 | 40.8 | Present study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueira Mancilha Nogueira, J.; Alves Silva, J.P.; Mussatto, S.I.; Melo Carneiro, L. Synthesis and Application of Heterogeneous Catalysts Based on Heteropolyacids for 5-Hydroxymethylfurfural Production from Glucose. Energies 2020, 13, 655. https://doi.org/10.3390/en13030655
Siqueira Mancilha Nogueira J, Alves Silva JP, Mussatto SI, Melo Carneiro L. Synthesis and Application of Heterogeneous Catalysts Based on Heteropolyacids for 5-Hydroxymethylfurfural Production from Glucose. Energies. 2020; 13(3):655. https://doi.org/10.3390/en13030655
Chicago/Turabian StyleSiqueira Mancilha Nogueira, Jéssica, João Paulo Alves Silva, Solange I. Mussatto, and Livia Melo Carneiro. 2020. "Synthesis and Application of Heterogeneous Catalysts Based on Heteropolyacids for 5-Hydroxymethylfurfural Production from Glucose" Energies 13, no. 3: 655. https://doi.org/10.3390/en13030655
APA StyleSiqueira Mancilha Nogueira, J., Alves Silva, J. P., Mussatto, S. I., & Melo Carneiro, L. (2020). Synthesis and Application of Heterogeneous Catalysts Based on Heteropolyacids for 5-Hydroxymethylfurfural Production from Glucose. Energies, 13(3), 655. https://doi.org/10.3390/en13030655