HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues
Abstract
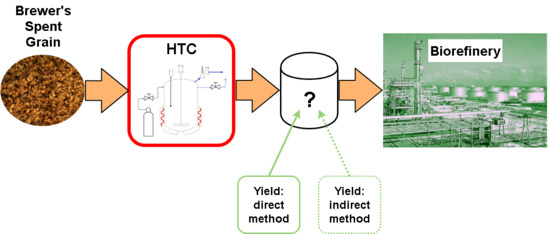
:1. Introduction
2. Materials and Methods
2.1. Characterisation of the Main Product–Beer
2.2. Characterisation of By-Product–Spent Grain before and after Carbonisation
2.3. Beer Brewing–Experimental Procedure and Description of the Experimental Setup
2.4. Hydrothermal Carbonisation–Experimental Rig and Characterisation of the Process
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bentzen, J.; Smith, V. Structural Changes in the Consumption of Beer, Wine and Spirits in OECD Countries from 1961 to 2014. Beverages 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Özvural, E.B.; Vural, H.; Gökbulut, İ.; Özboy-Özbaş, Ö. Utilization of brewer’s spent grain in the production of Frankfurters. Int. J. Food Sci. Technol. 2009, 44, 1093–1099. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P. The effect of different enzymes on the quality of high-fibre enriched brewer’s spent grain breads. Food Chem. 2008, 110, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Combest, S.; Warren, C. Perceptions of college students in consuming whole grain foods made with Brewers ’ Spent Grain. Food Sci. Nutr. 2019, 7, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandio, G.; Amoriello, T.; Carbone, K.; Fedrizzi, M.; Monteleone, A.; Tarangioli, S.; Pagano, M. Increasing the value of spent grain from craft microbreweries for energy purposes. Chem. Eng. Trans. 2017, 58, 487–492. [Google Scholar] [CrossRef]
- Jackowski, M.; Semba, D.; Trusek, A.; Wnukowski, M.; Niedzwiecki, L.; Baranowski, M.; Krochmalny, K.; Pawlak-Kruczek, H. Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Arauzo, P.; Olszewski, M.; Kruse, A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Riaño, P.; Sanz Diez, M.T.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of polyphenols from brewer’s spent grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid state fermentation of Brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17. Fermentation 2019, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water-organic solvent extraction of phenolic antioxidants from brewers’ spent grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, neutral, and polar lipids of brewing ingredients, by-products and beer: Evaluation of antithrombotic activities. Foods 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Arauzo, P.J.; Meza Zavala, M.F.; Cao, Z.; Olszewski, M.P.; Kruse, A. Towards the properties of different biomass-derived proteins via various extraction methods. Molecules 2020, 25, 488. [Google Scholar] [CrossRef] [Green Version]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Biosourced disposable trays made of brewer’s spent grain and potato starch. Polymers 2019, 11, 923. [Google Scholar] [CrossRef] [Green Version]
- Zedler, Ł.; Colom, X.; Cañavate, J.; Saeb, M.R.; Haponiuk, J.T.; Formela, K. Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber. Polymers 2020, 12, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbir, S.; Goksungur, Y. Natural red pigment production by monascus purpureus in submerged fermentation systems using a food industry waste: Brewer’s spent grain. Foods 2019, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Amoriello, T.; Fiorentino, S.; Vecchiarelli, V.; Pagano, M. Evaluation of spent grain biochar impact on hop (Humulus lupulus L.) growth by multivariate image analysis. Appl. Sci. 2020, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from brewers’ spent grain: A green and low-cost smart material to modify screen-printed electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Enweremadu, C.; Waheed, M.A.; Adekunle, A.A.; Adeala, A. The Energy Potential of Brewer’s Spent Grain for Breweries in Nigeria. Eng. Appl. Sci. 2008, 3, 175–177. [Google Scholar]
- Liguori, R.; Soccol, C.R.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Faraco, V. Second generation ethanol production from brewers’ spent grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonisation of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of wet and high ash biomass: Wet torrefaction—A review. J. Power Technol. 2017, 97, 354–369. [Google Scholar]
- Codignole Luz, F.; Volpe, M.; Fiori, L.; Manni, A.; Cordiner, S.; Mulone, V.; Rocco, V. Spent coffee enhanced biomethane potential via an integrated hydrothermal carbonization-anaerobic digestion process. Bioresour. Technol. 2018, 256, 102–109. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef]
- Gao, L.; Volpe, M.; Lucian, M.; Fiori, L.; Goldfarb, J.L. Does Hydrothermal Carbonization as a Biomass Pretreatment Reduce Fuel Segregation of Coal-Biomass Blends During Oxidation? Energy Convers. Manag. 2018, 181, 93–104. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef]
- Tungal, R.; Shende, R.V. Hydrothermal liquefaction of pinewood (Pinus ponderosa) for H2, biocrude and bio-oil generation. Appl. Energy 2014, 134, 401–412. [Google Scholar] [CrossRef]
- Nan, W.; Shende, A.R.; Shannon, J.; Shende, R.V. Insight into Catalytic Hydrothermal Liquefaction of Cardboard for Biofuels Production. Energy Fuels 2016, 30, 4933–4944. [Google Scholar] [CrossRef]
- Shende, R.; Tungal, R. Subcritical Aqueous Phase Reforming of Wastepaper for Biocrude and H2 Generation. Energy Fuels 2013, 27, 3194–3203. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour. Technol. 2011, 102, 7595–7598. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus-Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass–Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A.; Defersha, F. Mechanical and alkaline hydrothermal treated corn residue conversion in to bioenergy and biofertilizer: A resource recovery concept. Energies 2018, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, D.; Kruse, A.; Sauer, J.; Vetter, P. Sucrose is a promising feedstock for the synthesis of the platform chemical hydroxymethylfurfural. Energies 2018, 11, 645. [Google Scholar] [CrossRef] [Green Version]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards biochar and hydrochar engineering-influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Zevaco, T.A. Properties of hydrochar as function of feedstock, reaction conditions and post-treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Persson, H.; Yang, W.; Jönsson, P.G. Pyrolysis study of hydrothermal carbonization-treated digested sewage sludge using a Py-GC/MS and a bench-scale pyrolyzer. Fuel 2019, 262, 116335. [Google Scholar] [CrossRef]
- Weber, K.; Heuer, S.; Quicker, P.; Li, T.; Løvås, T.; Scherer, V. An Alternative Approach for the Estimation of Biochar Yields. Energy Fuels 2018, 32, 9506–9512. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Sepúlveda, F.J.; Montero, I.; Rojas, C.V. Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467. [Google Scholar] [CrossRef] [Green Version]
- Dudek, M.; Świechowski, K.; Manczarski, P.; Koziel, J.A.; Białowiec, A. The effect of biochar addition on the biogas production kinetics from the anaerobic digestion of brewers’ spent grain. Energies 2019, 12, 1518. [Google Scholar] [CrossRef] [Green Version]
- Poerschmann, J.; Weiner, B.; Wedwitschka, H.; Baskyr, I.; Koehler, R.; Kopinke, F.D. Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresour. Technol. 2014, 164, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.P.; Arauzo, P.J.; Wądrzyk, M.; Kruse, A. Py-GC-MS of hydrochars produced from brewer’s spent grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.M.; Kruse, A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 260, 121101. [Google Scholar] [CrossRef]
- European Comitte for Standardisation (CEN). EN ISO 18122:2015 Solid Biofuels—Determination of Ash Content; BSI Standards Limited: London, UK, 2015; ISBN 978 0 580 81427 3. [Google Scholar]
- European Comitte for Standardisation (CEN). EN 15148:2009 Solid Biofuels—Determination of the Content of Volatile Matter; BSI Standards Limited: London, UK, 2009; ISBN 978 0 580 66694 0. [Google Scholar]
- European Comitte for Standardisation (CEN). EN ISO 16948:2015 Determination of Total Content of Carbon, Hydrogen and Nitrogen—Instrumental Methods; BSI Standards Limited: London, UK, 2015; ISBN 978 0 580 81463 1. [Google Scholar]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Brown, A.B.; Mudhoo, A.; Martinez, J.; Timko, M.T.; Rostagno, M.A.; Forster-Carneiro, T. Applications of subcritical and supercritical water conditions for extraction, hydrolysis, gasification, and carbonization of biomass: A critical review. Biofuel Res. J. 2017, 4, 611–626. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Krochmalny, K.; Mościcki, K.; Zgóra, J.; Czerep, M.; Ostrycharczyk, M.; Niedźwiecki, Ł. Torrefaction of Various Types of Biomass in Laboratory Scale, Batch-Wise Isothermal Rotary Reactor and Pilot Scale, Continuous Multi-Stage Tape Reactor. Eng. Prot. Environ. 2017, 20, 457–472. [Google Scholar] [CrossRef]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of biomass: Dry torrefaction and pelletization-a review. J. Power Technol. 2014, 94, 233–249. [Google Scholar]
- Pawlak-Kruczek, H.; Krochmalny, K.K.; Wnukowski, M.; Niedzwiecki, L. Slow pyrolysis of the sewage sludge with additives: Calcium oxide and lignite. J. Energy Resour. Technol. 2018, 140, 062206. [Google Scholar] [CrossRef]
- Liaw, S.B.; Wu, H. A New Method for Direct Determination of Char Yield during Solid Fuel Pyrolysis in Drop-Tube Furnace at High Temperature and Its Comparison with Ash Tracer Method. Energy Fuels 2019, 33, 1509–1517. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Gu, J.H.; Lim, Y.; Oh, S.C. Effect of Co-Torrefaction on the Properties of Sewage Sludge and Waste Wood to Enhance Solid Fuel Qualities. J. Residuals Sci. Technol. 2017, 14, 23–36. [Google Scholar] [CrossRef]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Thompson Witrick, K.; Duncan, S.; Hurley, K.; O’Keefe, S. Acid and Volatiles of Commercially-Available Lambic Beers. Beverages 2017, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Bach, Q.V.; Tran, K.Q.; Skreiberg, O.; Khalil, R.A.; Phan, A.N. Effects of wet torrefaction on reactivity and kinetics of wood under air combustion conditions. Fuel 2014, 137, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Puccini, M.; Ceccarini, L.; Antichi, D.; Seggiani, M.; Tavarini, S.; Latorre, M.H.; Vitolo, S. Hydrothermal carbonization of municipal woody and herbaceous prunings: Hydrochar valorisation as soil amendment and growth medium for horticulture. Sustainability 2018, 10, 846. [Google Scholar] [CrossRef] [Green Version]
- Parmar, K.R.; Ross, A.B. Integration of hydrothermal carbonisation with anaerobic digestion; Opportunities for valorisation of digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef] [Green Version]
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory studies on the influence of biomass particle size on pyrolysis and combustion using TG GC/MS. Fuel 2019, 252, 635–645. [Google Scholar] [CrossRef]
- Broch, A.; Jena, U.; Hoekman, S.K.; Langford, J. Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 2014, 7, 62–79. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 2018, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Louwes, A.C.; Basile, L.; Yukananto, R.; Bhagwandas, J.C.; Bramer, E.A.; Brem, G. Torrefied biomass as feed for fast pyrolysis: An experimental study and chain analysis. Biomass Bioenergy 2017, 105, 116–126. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.A.; Dierkes, W.; Akkerman, R.; Brem, G. A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Jin, Y.; Du, J.; Zhang, K.; Zhao, D. Effects of wheat protein compositions on malt quality. Qual. Assur. Saf. Crop. Foods 2014, 6, 73–80. [Google Scholar] [CrossRef]
- Magliano, P.N.; Prystupa, P.; Gutiérrez-Boem, F.H. Protein content of grains of different size fractions in malting barley. J. Inst. Brew. 2014, 120, 347–352. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Alcázar, A.; Pablos, F.; Martín, M.J.; González, A.G. Multivariate characterisation of beers according to their mineral content. Talanta 2002, 57, 45–52. [Google Scholar] [CrossRef]
- Beidaghy Dizaji, H.; Faraji Dizaji, F.; Bidabadi, M. Determining thermo-kinetic constants in order to classify explosivity of foodstuffs. Combust. Explos. Shock Waves 2014, 50, 454–462. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Arauzo, P.J.; Maziarka, P.A.; Ronsse, F.; Kruse, A. Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains. Catalysts 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Gui, Y.; Li, J.; Zhu, Y.; Guo, L. Roles of four enzyme crosslinks on structural, thermal and gel properties of potato proteins. LWT - Food Sci. Technol. 2020, 123, 109116. [Google Scholar] [CrossRef]
- Pyle, D.L.; Zaror, C.A. Heat transfer and kinetics in the low temperature pyrolysis of solids. Chem. Eng. Sci. 1984, 39, 147–158. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal carbonization of yard waste for solid bio-fuel production: Study on combustion kinetic, energy properties, grindability and flowability of hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, H.; Liu, G.H.; Mei, C.L.; Ji, Y. Qualitative Prediction of Yeast Growth Process Based on Near Infrared Spectroscopy. Chin. J. Anal. Chem. 2017, 45, 1137–1141. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Koppejan, J.; Sokhansanj, S.; Melin, S.; Madrali, S. Status Overview of Torrefaction Technologies; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Butler, E.; Devlin, G.; Meier, D.; McDonnell, K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sustain. Energy Rev. 2011, 15, 4171–4186. [Google Scholar] [CrossRef] [Green Version]
- Weiner, B.; Poerschmann, J.; Wedwitschka, H.; Koehler, R.; Kopinke, F.-D. Influence of Process Water Reuse on the Hydrothermal Carbonization of Paper. ACS Sustain. Chem. Eng. 2014, 2, 2165–2171. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Mumme, J. Anaerobic Digestion of Waste Water from Hydrothermal Carbonization of Corn Silage. Appl. Bioenergy 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodríguez, J.J.; Borja, R.; De la Rubia, M.A. Anaerobic Co-digestion of the Organic Fraction of Municipal Solid Waste and the Liquid Fraction From the Hydrothermal Carbonization of Industrial Sewage Sludge Under Thermophilic Conditions. Front. Sustain. Food Syst. 2018, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Svensson, K.; Kjørlaug, O.; Higgins, M.J.; Linjordet, R.; Horn, S.J. Post-anaerobic digestion thermal hydrolysis of sewage sludge and food waste: Effect on methane yields, dewaterability and solids reduction. Water Res. 2018, 132, 158–166. [Google Scholar] [CrossRef] [PubMed]














| Sample | HTC Temperature | Residence Time | Average Heating Rate 1 |
|---|---|---|---|
| °C | min | °C / min | |
| Barley-raw spent grain | - | - | - |
| Barley, 180 °C, 10 min | 180 | 10 | 3.7 |
| Barley, 200 °C, 10 min | 200 | 10 | 2.9 |
| Barley, 200 °C, 60 min | 200 | 60 | 3.1 |
| Wheat-raw spent grain | - | - | - |
| Wheat, 200 °C, 10 min | 200 | 10 | 2.7 |
| Wheat, 200 °C, 60 min | 200 | 60 | 2.1 |
| Wheat, 200 °C, 120 min | 200 | 120 | 2.4 |
| Compound | Barley-Based Beer | Wheat-Based Beer | Primátor Weizen |
|---|---|---|---|
| a.u. 1 | a.u. 1 | a.u. 1 | |
| 1-Butanol, 3-methyl- / 1-Pentanol | 781,362 | 3,160,527 | n.d. 2 |
| 2-Propanone, 1-hydroxy | 245,906 | 202,954 | 274,030 |
| Acetic acid / Ammonium acetate | 3,154,572 | 2,101,582 | 802,539 |
| 2,3-Butanediol / 2,3-Butanediol, [R-(R*,R*)]- / 2,3-Butanediol, [S-(R*,R*)] 3 | 4,012,472 | 643,145 | 3,721,274 |
| 2,3-Butanediol / 2,3-Butanediol, [R-(R*,R*)]- / 2,3-Butanediol, [S-(R*,R*)] 3 | 2,008,409 | 493,655 | 1,512,920 |
| 2-Furanmethanol / 3-Furanmethanol / Methylenecyclopropanecarboxylic acid | 2,432,529 | 322,155 | 1,307,080 |
| Phenylethyl Alcohol / Hydrazine, (phenylmethyl)- | 2,763,552 | 6,186,847 | 3,200,241 |
| Maltol | 2,689,918 | 591,574 | 4,307,609 |
| (S)-(+)-2′,3′-Dideoxyribonolactone / 5-Hydroxymethyldihydrofuran-2-one | 650,481 | 92,640 | n.d. |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 208,087 | n.d. | 92,069 |
| Glycerin | 29,365,286 | 31,641,608 | 30,998,826 |
| (S)-(+)-2′,3′-Dideoxyribonolactone / 5-Hydroxymethyldihydrofuran-2-one | 40,841 | 85,481 | n.d. |
| 5-Hydroxymethylfurfural | 470,982 | n.d. | n.d. |
| 2(3H)-Furanone, dihydro-4-hydroxy- | 240,062 | n.d. | n.d. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackowski, M.; Niedzwiecki, L.; Lech, M.; Wnukowski, M.; Arora, A.; Tkaczuk-Serafin, M.; Baranowski, M.; Krochmalny, K.; Veetil, V.K.; Seruga, P.; et al. HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues. Energies 2020, 13, 2058. https://doi.org/10.3390/en13082058
Jackowski M, Niedzwiecki L, Lech M, Wnukowski M, Arora A, Tkaczuk-Serafin M, Baranowski M, Krochmalny K, Veetil VK, Seruga P, et al. HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues. Energies. 2020; 13(8):2058. https://doi.org/10.3390/en13082058
Chicago/Turabian StyleJackowski, Mateusz, Lukasz Niedzwiecki, Magdalena Lech, Mateusz Wnukowski, Amit Arora, Monika Tkaczuk-Serafin, Marcin Baranowski, Krystian Krochmalny, Vivek K. Veetil, Przemysław Seruga, and et al. 2020. "HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues" Energies 13, no. 8: 2058. https://doi.org/10.3390/en13082058
APA StyleJackowski, M., Niedzwiecki, L., Lech, M., Wnukowski, M., Arora, A., Tkaczuk-Serafin, M., Baranowski, M., Krochmalny, K., Veetil, V. K., Seruga, P., Trusek, A., & Pawlak-Kruczek, H. (2020). HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues. Energies, 13(8), 2058. https://doi.org/10.3390/en13082058