Effects of Impurities on Pre-Doped and Post-Doped Membranes for High Temperature PEM Fuel Cell Stacks
Abstract
:1. Introduction
2. Methodology
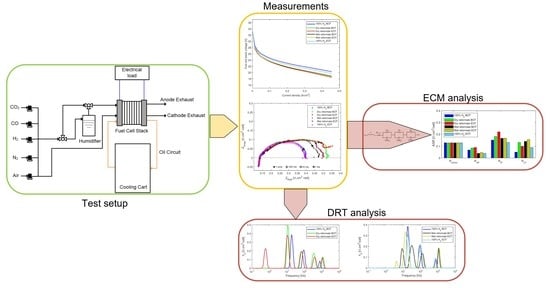
2.1. Experimental Setup
2.2. Test Procedures
2.3. Data Analysis
3. Results
3.1. Nitrogen Dilution
3.2. Poisoning Effects of Dry and Wet Reformate Impurities
3.2.1. Poisoning Effects on an HT-PEMFC Stack with Post-Doped MEAs
3.2.2. Poisoning Effects on an HT-PEMFC Stack with Pre-Doped MEAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOT | Beginning of test |
| CPE | Constant phase element |
| DRT | Distribution of relaxation time |
| ECM | Equivalent circuit model |
| EIS | Electrochemical impedance spectroscopy |
| EOT | End of test |
| GDL | Gas diffusion layer |
| HOR | Hydrogen oxidation reaction |
| HT-PEMFC | High temperature polymer electrolyte membrane fuel cell |
| LT-PEMFC | Low temperature polymer electrolyte membrane fuel cell |
| MEA | Membrane electrode assembly |
| MPL | Mesoporous layer |
| ORR | Oxygen reduction reaction |
| PBI | Polybenzimidazole |
| PFSA | Perfluorosulfonic acid |
| PTFE | Polytetrafluoroethylene |
References
- Ho, J.C.; Saw, E.C.; Lu, L.Y.; Liu, J.S. Technological barriers and research trends in fuel cell technologies: A citation network analysis. Technol. Forecast. Soc. Chang. 2014, 82, 66–79. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Qu, K.; Zhang, Z.; He, P. Mechanical behavior of a hydrated perfluorosulfonic acid membrane at meso and nano scales. RSC Adv. 2019, 9, 9594–9603. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, A.; Messana, A.; Airale, A.G.; Sisca, L.; de Carvalho Pinheiro, H.; Zevola, F.; Carello, M. Nafionr tubing humidification system for polymer electrolyte membrane fuel cells. Energies 2019, 12, 1773. [Google Scholar] [CrossRef] [Green Version]
- Carello, M.; De Vita, A.; Ferraris, A. Method for Increasing the Humidity in Polymer Electrolyte Membrane Fuel Cell. Fuel Cells 2016, 16, 157–164. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Y.; Lu, S.; Jiang, S.P. High Temperature Polymer Electrolyte Membrane Fuel Cells for Integrated Fuel Cell—Methanol Reformer Power Systems: A Critical Review. Adv. Sustain. Syst. 2018, 2, 1700184. [Google Scholar] [CrossRef]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Büsselmann, J.; Rastedt, M.; Klicpera, T.; Reinwald, K.; Schmies, H.; Dyck, A.; Wagner, P. Analysis of HT-PEM MEAs’ Long-Term Stabilities. Energies 2020, 13, 567. [Google Scholar] [CrossRef] [Green Version]
- Søndergaard, T.; Cleemann, L.N.; Becker, H.; Steenberg, T.; Hjuler, H.A.; Seerup, L.; Li, Q.; Jensen, J.O. Long-Term Durability of PBI-Based HT-PEM Fuel Cells: Effect of Operating Parameters. J. Electrochem. Soc. 2018, 165, F3053–F3062. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Andreasen, S.J.; Kær, S.K.; Park, J.O. Experimental investigation of carbon monoxide poisoning effect on a PBI/H3PO4 high temperature polymer electrolyte membrane fuel cell: Influence of anode humidification and carbon dioxide. Int. J. Hydrog. Energy 2015, 40, 14932–14941. [Google Scholar] [CrossRef]
- Zhou, F.; Singdeo, D.; Kær, S.K. Investigation of the Effect of Humidity Level of H2 on Cell Performance of a HT-PEM Fuel Cell. Fuel Cells 2019, 19, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, H.; Kim, D.K.; Lee, W.J.; Choi, I.; Kim, H.J.; Kim, S.K. Performance deterioration and recovery in high-temperature polymer electrolyte membrane fuel cells: Effects of deliquescence of phosphoric acid. Int. J. Hydrog. Energy 2020, 45, 32844–32855. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J.; Oluf, J.; Savinell, R.F.; Bjerrum, N.J.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Study of the oxygen reduction reaction (ORR) at Pt interfaced with phosphoric acid doped polybenzimidazole at elevated temperature and low relative humidity. Electrochim. Acta 2006, 51, 3914–3923. [Google Scholar] [CrossRef]
- Strmcnik, D.; Escudero-Escribano, M.; Kodama, K.; Stamenkovic, V.R.; Cuesta, A.; Markovií, N.M. Enhanced electrocatalysis of the oxygen reduction reaction based on patterning of platinum surfaces with cyanide. Nat. Chem. 2010, 2, 880–885. [Google Scholar] [CrossRef]
- Myles, T.; Bonville, L.; Maric, R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts 2017, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Tahrim, A.A.; Amin, I.N.H.M. Advancement in Phosphoric Acid Doped Polybenzimidazole Membrane for High Temperature PEM Fuel Cells: A Review. J. Appl. Membr. Sci. Technol. 2018, 23, 37–62. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.J.; Baurmeister, J. Properties of high-temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]
- Vang, J.R.; Andreasen, S.J.; Araya, S.S.; Kær, S.K. Comparative study of the break in process of post doped and sol–gel high temperature proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2014, 39, 14959–14968. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zholobko, O.; Wu, X.F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2020, 14, 135. [Google Scholar] [CrossRef]
- Bevilacqua, N.; Asset, T.; Schmid, M.; Markötter, H.; Manke, I.; Atanassov, P.; Zeis, R. Impact of catalyst layer morphology on the operation of high temperature PEM fuel cells. J. Power Sources Adv. 2021, 7, 100042. [Google Scholar] [CrossRef]
- Kim, S.; Myles, T.D.; Kunz, H.; Kwak, D.; Wang, Y.; Maric, R. The effect of binder content on the performance of a high temperature polymer electrolyte membrane fuel cell produced with reactive spray deposition technology. Electrochim. Acta 2015, 177, 190–200. [Google Scholar] [CrossRef]
- Bevilacqua, N.; George, M.G.; Galbiati, S.; Bazylak, A.; Zeis, R. Phosphoric Acid Invasion in High Temperature PEM Fuel Cell Gas Diffusion Layers. Electrochim. Acta 2017, 257, 89–98. [Google Scholar] [CrossRef]
- Chevalier, S.; Fazeli, M.; Mack, F.; Galbiati, S.; Manke, I.; Bazylak, A.; Zeis, R. Role of the microporous layer in the redistribution of phosphoric acid in high temperature PEM fuel cell gas diffusion electrodes. Electrochim. Acta 2016, 212, 187–194. [Google Scholar] [CrossRef]
- Rosli, R.; Sulong, A.; Daud, W.; Zulkifley, M.; Husaini, T.; Rosli, M.; Majlan, E.; Haque, M. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrog. Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Martin, S.; Garcia-Ybarra, P.L.; Castillo, J.L. Ten-fold reduction from the state-of-the-art platinum loading of electrodes prepared by electrospraying for high temperature proton exchange membrane fuel cells. Electrochem. Commun. 2018, 93, 57–61. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, W.; Ma, Q.; Xu, Q.; Pasupathi, S.; Su, H. Achieving high Pt utilization and superior performance of high temperature polymer electrolyte membrane fuel cell by employing low-Pt-content catalyst and microporous layer free electrode design. J. Power Sources 2019, 426, 124–133. [Google Scholar] [CrossRef]
- Kannan, A.; Li, Q.; Cleemann, L.N.; Jensen, J.O. Acid Distribution and Durability of HT-PEM Fuel Cells with Different Electrode Supports. Fuel Cells 2018, 18, 103–112. [Google Scholar] [CrossRef]
- de Beer, C.; Barendse, P.S.; Pillay, P.; Bullecks, B.; Rengaswamy, R. Electrical Circuit Analysis of CO Poisoning in High-Temperature PEM Fuel Cells for Fault Diagnostics and Mitigation. IEEE Trans. Ind. Appl. 2015, 51, 619–630. [Google Scholar] [CrossRef]
- Malinowski, M.; Iwan, A.; Pasciak, G.; Parafiniuk, K.; Gorecki, L.; Pa ciak, G.; Parafiniuk, K.; Gorecki, L. Synthesis and characterization of para- and meta-polybenzimidazoles for high-temperature proton exchange membrane fuel cells. High Perform. Polym. 2014, 26, 436–444. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Mesquita, T.J.; Devos, O.; Tribollet, B.; Roche, V.; Nogueira, R.P. On the intrinsic coupling between constant-phase element parameters α and Q in electrochemical impedance spectroscopy. Electrochim. Acta 2012, 72, 172–178. [Google Scholar] [CrossRef]
- Shoar Abouzari, M.R.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements. Solid State Ion. 2009, 180, 922–927. [Google Scholar] [CrossRef]
- Gomadam, P.M.; Weidner, J.W. Analysis of electrochemical impedance spectroscopy in proton exchange membrane fuel cells. Int. J. Energy Res. 2005, 29, 1133–1151. [Google Scholar] [CrossRef]
- Cooper, K.; Smith, M. Electrical test methods for on-line fuel cell ohmic resistance measurement. J. Power Sources 2006, 160, 1088–1095. [Google Scholar] [CrossRef]
- Jeppesen, C.; Araya, S.S.; Sahlin, S.L.; Thomas, S.; Andreasen, S.J.; Kær, S.K. Fault detection and isolation of high temperature proton exchange membrane fuel cell stack under the influence of degradation. J. Power Sources 2017, 359, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Araya, S.S.; Frensch, S.H.; Steenberg, T.; Kær, S.K. Hydrogen mass transport resistance changes in a high temperature polymer membrane fuel cell as a function of current density and acid doping. Electrochim. Acta 2019, 317, 521–527. [Google Scholar] [CrossRef]
- Weiß, A.; Schindler, S.; Galbiati, S.; Danzer, M.A.; Zeis, R. Distribution of Relaxation Times Analysis of High-Temperature PEM Fuel Cell Impedance Spectra. Electrochim. Acta 2017, 230, 391–398. [Google Scholar] [CrossRef]
- Schenk, A.; Grimmer, C.; Perchthaler, M.; Weinberger, S.; Pichler, B.; Heinzl, C.; Scheu, C.; Mautner, F.A.; Bitschnau, B.; Hacker, V. Platinum-cobalt catalysts for the oxygen reduction reaction in high temperature proton exchange membrane fuel cells—Long term behavior under ex-situ and in-situ conditions. J. Power Sources 2014, 266, 313–322. [Google Scholar] [CrossRef]
- Schindler, S.; Weiss, A.; Galbiati, S.; Mack, F.; Danzer, M.A.; Zeis, R. Identification of Polarization Losses in High-Temperature PEM Fuel Cells by Distribution of Relaxation Times Analysis. ECS Trans. 2016, 75, 45–53. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Heinzmann, M.; Weber, A.; Ivers-Tiffée, E. Advanced impedance study of polymer electrolyte membrane single cells by means of distribution of relaxation times. J. Power Sources 2018, 402, 24–33. [Google Scholar] [CrossRef]
- Simon Araya, S.; Zhou, F.; Lennart Sahlin, S.; Thomas, S.; Jeppesen, C.; Knudsen Kær, S. Fault Characterization of a Proton Exchange Membrane Fuel Cell Stack. Energies 2019, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Lai, W.H.; Chen, Y.K.; Su, S.S. Characteristic studies of a PBI/H3PO4 high temperature membrane PEMFC under simulated reformate gases. Int. J. Hydrog. Energy 2014, 39, 13757–13762. [Google Scholar] [CrossRef]
- Andreasen, S.J.; Vang, J.R.; Kær, S.K. High temperature PEM fuel cell performance characterisation with CO and CO2 using electrochemical impedance spectroscopy. Int. J. Hydrog. Energy 2011, 36, 9815–9830. [Google Scholar] [CrossRef]
- Daletou, M.K.; Kallitsis, J.K.; Voyiatzis, G.; Neophytides, S.G. The interaction of water vapors with H3PO4 imbibed electrolyte based on PBI/polysulfone copolymer blends. J. Membr. Sci. 2009, 326, 76–83. [Google Scholar] [CrossRef]
- Du, B.; Pollard, R.; Elter, J.F.; Ramani, M. Performance and Durability of a Polymer Electrolyte Fuel Cell Operating with Reformate: Effects of CO, CO2, and Other Trace Impurities. In Polymer Electrolyte Fuel Cell Durability; Springer: New York, NY, USA, 2009; pp. 341–366. [Google Scholar]
- Reimer, U.; Ehlert, J.; Janßen, H.; Lehnert, W. Water distribution in high temperature polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2016, 41, 1837–1845. [Google Scholar] [CrossRef]
- Bevilacqua, N.; Schmid, M.; Zeis, R. Understanding the role of the anode on the polarization losses in high-temperature polymer electrolyte membrane fuel cells using the distribution of relaxation times analysis. J. Power Sources 2020, 471, 228469. [Google Scholar] [CrossRef]









| Test Step | Anode Gas Composition | Duration |
|---|---|---|
| Break-in | 100% H2 | 50 h |
| Nitrogen dilution | 100% H2, 31.7% N2 | 24 h |
| Dry reformate | 68.3% H2, 0.9% CO, 21.8% CO2, 9% N2 | 24 h |
| Air-bleed | 2% air, 98% H2 | 5 min |
| Wet reformate | 68.3% H2, 0.9% CO, 21.8% CO2, 9% H2O | 24 h |
| Air-bleed | 2% air, 98% H2 | 5 min |
| End of test recovery | 100% H2 | 24 h |
| Test | Stack with Post-Doped MEAs | Stack with Pre-Doped MEAs | ||
|---|---|---|---|---|
| ECM | DRT | ECM | DRT | |
| N2 dilution | R, R, | 4 peaks ↑ | R, R | 3 peaks |
| R, R | R, R | peaks < 100 Hz ↓ | ||
| peak at 1 kHz ↑ | ||||
| Dry reformate | R, R, R | 4–5 peaks ↑ | R, R, R | 3–5 peaks ↑ |
| R | R | peak below 1 Hz | ||
| Wet reformate | R, R, R | 4–5 peaks ↑ | R R | 4 peaks |
| R | R, R | peaks < 100 Hz ↓ | ||
| peak at 1 kHz ↑ | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon Araya, S.; Thomas, S.; Lotrič, A.; Lennart Sahlin, S.; Liso, V.; Andreasen, S.J. Effects of Impurities on Pre-Doped and Post-Doped Membranes for High Temperature PEM Fuel Cell Stacks. Energies 2021, 14, 2994. https://doi.org/10.3390/en14112994
Simon Araya S, Thomas S, Lotrič A, Lennart Sahlin S, Liso V, Andreasen SJ. Effects of Impurities on Pre-Doped and Post-Doped Membranes for High Temperature PEM Fuel Cell Stacks. Energies. 2021; 14(11):2994. https://doi.org/10.3390/en14112994
Chicago/Turabian StyleSimon Araya, Samuel, Sobi Thomas, Andrej Lotrič, Simon Lennart Sahlin, Vincenzo Liso, and Søren Juhl Andreasen. 2021. "Effects of Impurities on Pre-Doped and Post-Doped Membranes for High Temperature PEM Fuel Cell Stacks" Energies 14, no. 11: 2994. https://doi.org/10.3390/en14112994
APA StyleSimon Araya, S., Thomas, S., Lotrič, A., Lennart Sahlin, S., Liso, V., & Andreasen, S. J. (2021). Effects of Impurities on Pre-Doped and Post-Doped Membranes for High Temperature PEM Fuel Cell Stacks. Energies, 14(11), 2994. https://doi.org/10.3390/en14112994