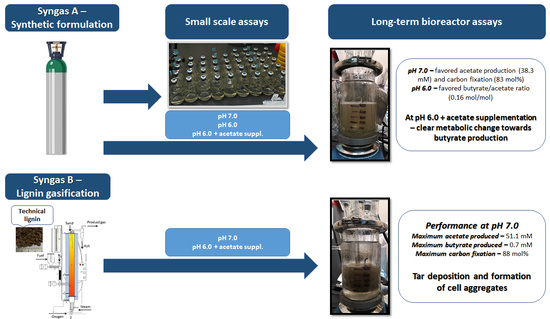
Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Culture Media and Syngas Composition
2.2. Experimental Set-Up
2.3. Analytical Methods
2.4. Calculations
3. Results and Discussion
3.1. Effect of Varying pH and Medium Supplementation on Syngas Bioconversion by B. methylotrophicum
3.2. Long-Term Synthetic Syngas Bioconversion by B. methylotrophicum
3.3. Long-Term Lignin Syngas Bioconversion by B. methylotrophicum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, C. Distribution of the Bio-Based Industry in the EU; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Wen, J.-L.; Wang, H.-M.; Ma, C.-Y.; Yuan, T.-Q.; Sun, R.-C. Value-added products from lignin: Isolation, characterization and applications. In Biomass, Biofuels, Biochemicals: Lignin Biorefinery; Bhaskar, T., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–55. ISBN 9780128202944. [Google Scholar]
- Liakakou, E.T.; Vreugdenhil, B.J.; Cerone, N.; Zimbardi, F.; Pinto, F.; André, R.; Marques, P.; Mata, R.; Girio, F. Gasification of Lignin-Rich Residues for the Production of Biofuels via Syngas Fermentation: Comparison of Gasification Technologies. Fuel 2019, 251, 580–592. [Google Scholar] [CrossRef]
- Whitty, K.J.; Zhang, H.R.; Eddings, E.G. Emissions from Syngas Combustion. Combust. Sci. Technol. 2008, 180, 1117–1136. [Google Scholar] [CrossRef]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J. Microbiology of Synthesis Gas Fermentation for Biofuel Production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Muthu Dineshkumar, R.; Angkayarkan Vinayakaselvi, M.; Ramanathan, A. Bio-Ethanol Production from Syngas-Derived Biomass: A Review. Mater. Today Proc. 2021, 46, 9989–9993. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Marques, P.; Mata, R.; Pacheco, M.; Moura, P.; Gírio, F. Production of Syngas Suitable to Be Used in Fermentation to Obtain Biochemical Added-Value Compounds. Chem. Eng. Trans. 2019, 76, 1399–1404. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting Gasification with Syngas Fermentation: Comparison of the Performance of Lignin and Beech Wood. Fuel 2021, 290, 120054. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-side Comparison of Clean and Biomass-Derived, Impurity-containing Syngas as Substrate for Acetogenic Fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Ramachandriya, K.D.; Wilkins, M.R.; Patil, K.N. Influence of Switchgrass Generated Producer Gas Pre-Adaptation on Growth and Product Distribution of Clostridium ragsdalei. Biotechnol. Bioprocess Eng. 2013, 18, 1201–1209. [Google Scholar] [CrossRef]
- Xu, D.; Lewis, R.S. Syngas Fermentation to Biofuels: Effects of Ammonia Impurity in Raw Syngas on Hydrogenase Activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.R.; Lewis, R.S. The Effects of Syngas Impurities on Syngas Fermentation to Liquid Fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Ahmed, A.; Lewis, R.S. Fermentation of Biomass-Generated Synthesis Gas: Effects of Nitric Oxide. Biotechnol. Bioeng. 2007, 97, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Datar, R.P.; Shenkman, R.M.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Fermentation of Biomass-Generated Producer Gas to Ethanol. Biotechnol. Bioeng. 2004, 86, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Effects of Biomass-Generated Producer Gas Constituents on Cell Growth, Product Distribution and Hydrogenase Activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 2006, 30, 665–672. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas Fermentation in a 100-L Pilot Scale Fermentor: Design and Process Considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zeikus, J.G.; Lynd, L.H.; Thompson, T.E.; Krzycki, J.A.; Weimer, P.J.; Hegge, P.W. Isolation and Characterization of a New, Methylotrophic, Acidogenic Anaerobe, the Marburg Strain. Curr. Microbiol. 1980, 3, 381–386. [Google Scholar] [CrossRef]
- Bertsch, J.; Müller, V. Bioenergetic Constraints for Conversion of Syngas to Biofuels in Acetogenic Bacteria. Biotechnol. Biofuels 2015, 8, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol Production from Biomass: Carbohydrate vs Syngas Fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Deshmukh, G.; Manyar, H. Production Pathways of Acetic Acid and Its Versatile Applications in the Food Industry. In Acetic Acid-Production and Applications in the Food Industry [Working Title]; IntechOpen: London, UK, 2020; ISBN 978-1-83881-182-2. [Google Scholar]
- Xu, Z.; Jiang, L. Butyric Acid. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon: Elmsford, NY, USA, 2011; Volume 3, pp. 235–243. ISBN 9780444640475. [Google Scholar]
- Kolesinska, B.; Fraczyk, J.; Binczarski, M.; Modelska, M.; Berlowska, J.; Dziugan, P.; Antolak, H.; Kaminski, Z.J.; Witonska, I.A.; Kregiel, D. Butanol Synthesis Routes for Biofuel Production: Trends and Perspectives. Materials 2019, 12, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, D.; Merkel, M.; Lilge, L.; Henkel, M.; Hausmann, R. From Acetate to Bio-Based Products: Underexploited Potential for Industrial Biotechnology. Trends. Biotechnol. 2021, 39, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; Jang, N.; Lee, M.; Kang, H.; Aslam, M.; Bazmi, A.A.; Chang, I.S. Bioreactors, Gas Delivery Systems and Supporting Technologies for Microbial Synthesis Gas Conversion Process. Bioresour. Technol. Rep. 2019, 7, 100207. [Google Scholar] [CrossRef]
- Arantes, A.L.; Moreira, J.P.C.; Diender, M.; Parshina, S.N.; Stams, A.J.M.; Alves, M.M.; Alves, J.I.; Sousa, D.Z. Enrichment of Anaerobic Syngas-Converting Communities and Isolation of a Novel Carboxydotrophic Acetobacterium wieringae Strain JM. Front. Microbiol. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diender, M.; Parera Olm, I.; Sousa, D.Z. Synthetic Co-Cultures: Novel Avenues for Bio-Based Processes. Curr. Opin. Biotechnol. 2021, 67, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Oswald, F.; Dörsam, S.; Veith, N.; Zwick, M.; Neumann, A.; Ochsenreither, K.; Syldatk, C. Sequential Mixed Cultures: From Syngas to Malic Acid. Front. Microbiol. 2016, 7, 891. [Google Scholar] [CrossRef] [PubMed]
- Gírio, F.; Marques, S.; Pinto, F.; Oliveira, A.C.; Costa, P.; Reis, A.; Moura, P. Biorefineries in the world. In Biorefineries. Lecture Notes in Energy; Rabaçal, M., Ferreira, A., Silva, C., Costa, M., Eds.; Springer: Cham, Switzerland, 2017; Volume 57, pp. 227–281. [Google Scholar] [CrossRef]
- Worden, R.M.; Grethlein, A.J.; Zeikus, J.G.; Datta, R. Butyrate Production from Carbon Monoxide by Butyribacterium methylotrophicum. App. Biochem. Biotechnol. 1989, 20–21, 687–698. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Latimer, G.W., Ed.; AOAC International: Rockville, MD, USA, 2019; Volumes 1–3, ISBN 0935584897. [Google Scholar]
- Ortigueira, J.; Alves, L.; Gouveia, L.; Moura, P. Third Generation Biohydrogen Production by Clostridium butyricum and Adapted Mixed Cultures from Scenedesmus obliquus Microalga Biomass. Fuel 2015, 153, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Infantes, A.; Kugel, M.; Neumann, A. Evaluation of Media Components and Process Parameters in a Sensitive and Robust Fed-Batch Syngas Fermentation System with Clostridium ljungdahlii. Fermentation 2020, 6, 61. [Google Scholar] [CrossRef]
- Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. H-B-E (Hexanol-Butanol-Ethanol) Fermentation for the Production of Higher Alcohols from Syngas/Waste Gas. J. Chem. Technol. Biotechnol. 2017, 92, 712–731. [Google Scholar] [CrossRef]
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The Effect of Syngas Composition on the Growth and Product Formation of Butyribacterium methylotrophicum. Enzym. Microb. Technol. 2007, 41, 362–367. [Google Scholar] [CrossRef]
- Park, S.; Yasin, M.; Jeong, J.; Cha, M.; Kang, H.; Jang, N.; Choi, I.G.; Chang, I.S. Acetate-Assisted Increase of Butyrate Production by Eubacterium limosum KIST612 during Carbon Monoxide Fermentation. Bioresour. Technol. 2017, 245, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Production of Medium-Chain Fatty Acids and Higher Alcohols by a Synthetic Co-Culture Grown on Carbon Monoxide or Syngas. Biotechnol. Biofuels 2016, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. NY Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Dürre, P. Butanol Formation from Gaseous Substrates. FEMS Microbiol. Lett. 2016, 363, 40. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Molitor, B.; Diender, M.; Sousa, D.Z.; Angenent, L.T. A Narrow pH Range Supports Butanol, Hexanol, and Octanol Production from Syngas in a Continuous Co-Culture of Clostridium ljungdahlii and Clostridium kluyveri with in-Line Product Extraction. Front. Microbiol. 2016, 7, 1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annous, B.A.; Shieh, J.-S.; Shen, G.-J.; Jain, M.K.; Zeikus, J.G. Regulation of Hydrogen Metabolism in Butyribacterium methylotrophicum by Substrate and pH. Appl. Microbiol. Biotechnol. 1996, 45, 804–810. [Google Scholar] [CrossRef]
- Grethlein, A.J.; Worden, R.M.; Jain, M.K.; Datta, R. Continuous Production of Mixed Alcohols and Acids from Carbon Monoxide. Appl. Biochem. Biotechnol. 1990, 24, 875–884. [Google Scholar] [CrossRef]
- Jaros, A.M.; Rova, U.; Berglund, K.A. Acetate Adaptation of Clostridia tyrobutyricum for Improved Fermentation Production of Butyrate. Springerplus 2013, 2, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueki, T.; Nevin, K.P.; Woodard, T.L.; Lovley, D.R. Converting Carbon Dioxide to Butyrate with an Engineered Strain of Clostridium ljungdahlii. mBio 2014, 5, e01636-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate Utilization and Butyryl Coenzyme A (CoA): Acetate-CoA Transferase in Butyrate-Producing Bacteria from the Human Large Intestine. Appl. Environ. Microb. 2002, 68, 5186–5190. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Naveira, Á.; Abubackar, H.N.; Veiga, M.C.; Kennes, C. Production of Chemicals from C1 Gases (CO, CO2) by Clostridium carboxidivorans. World J. Microbiol. Biotechnol. 2017, 33, 43. [Google Scholar] [CrossRef] [PubMed]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial Autoaggregation. AIMS Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Summers, R.; Cook, S. Development and Experimental Validation of the Composition and Treatability of a New Synthetic Bathroom Greywater (SynGrey). Environ. Sci. Water Res. Technol. 2017, 3, 1120–1131. [Google Scholar] [CrossRef]
- Abu Hassan, M.H.; Sher, F.; Fareed, B.; Ali, U.; Zafar, A.; Bilal, M.; Iqbal, H.M.N. Sustainable Hydrates for Enhanced Carbon Dioxide Capture from an Integrated Gasification Combined Cycle in a Fixed Bed Reactor. Ind. Eng. Chem. Res. 2021, 60, 11346–11356. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Zamri, N.M.; May, S.T.; Azizan, M.T.; Aqsha, A.; Sabzoi, N.; Sher, F. Effect of Acid Catalysts on Hydrothermal Carbonization of Malaysian Oil Palm Residues (Leaves, Fronds, and Shells) for Hydrochar Production. Biomass Convers. Biorefin. 2021. Available online: https://link.springer.com/article/10.1007%2Fs13399-020-01201-2 (accessed on 22 October 2021). [CrossRef]
 —Optical density at 600 nm;
—Optical density at 600 nm;  —acetate;
—acetate;  —butyrate;
—butyrate;  —CO;
—CO;  —H2;
—H2;  —CO2).
—CO2).
 —Optical density at 600 nm;
—Optical density at 600 nm;  —acetate;
—acetate;  —butyrate;
—butyrate;  —CO;
—CO;  —H2;
—H2;  —CO2).
—CO2).
 —pressure inside the bioreactor;
—pressure inside the bioreactor;  —Optical density at 600 nm;
—Optical density at 600 nm;  —acetate;
—acetate;  —butyrate; —pH set-point change).
—butyrate; —pH set-point change).
 —pressure inside the bioreactor;
—pressure inside the bioreactor;  —Optical density at 600 nm;
—Optical density at 600 nm;  —acetate;
—acetate;  —butyrate; —pH set-point change).
—butyrate; —pH set-point change).



| Syngas Compound | Syngas A | Syngas B † | Units |
|---|---|---|---|
| CO | 30.0 | 24.2 | vol% |
| CO2 | 20.0 | 16.5 | vol% |
| H2 | 30.0 | 23.9 | vol% |
| CH4 | - | 13.6 | vol% |
| N2 | 20.0 | 18.1 | vol% |
| O2 | - | 0.4 | vol% |
| C2H2 | - | nd ‡ | - |
| C2H4 | - | 3.0 | vol% |
| C2H6 | - | 0.1 | vol% |
| C3H6 | - | 300 | ppmV |
| C3H8 | - | 500 | ppmV |
| C4H10 | - | 300 | ppmV |
| NH3 | - | 760 | ppmV |
| H2S | - | 180 | ppmV |
| Parameter | pH 7.0 | pH 6.0 | pH 6.0 and NaAc 1 |
|---|---|---|---|
| Max. specific growth rate 2 (h−1) | 0.07 (R2 = 0.99) | 0.05 (R2 = 1.00) | 0.05 (R2 = 0.97) |
| Acetate (mM) | 29.0 ± 1.0 | 32.0 ± 1.2 | 20.2 ± 0.3 |
| Butyrate (mM) | 1.7 ± 0.4 | 0.9 ± 0.1 | 5.7 ± 0.1 |
| Butyrate/acetate produced (mol/mol) | 0.06 | 0.05 | 0.59 |
| Final pH | 6.6 ± 0.1 | 5.8 ± 0.0 | 6.0 ± 0.0 |
| Pressure after 120 h (kPa) 3 | 42.6 | 44.6 | 45.6 |
| CF (mol %) | 61 | 87 | 86 |
| Parameters. | pH 7.0 | pH 6.0 |
|---|---|---|
| Max. specific growth rate 1 (h−1) | 0.012 | 0.006 |
| Acetate (mM) 2 | 38.3 | 20.2 |
| Butyrate (mM) 2 | 2.0 | 1.2 |
| Butyrate/Acetate produced (mol/mol) | 0.05 | 0.16 |
| Min. pressure 3 (kPa) | 60.8 | 60.9 |
| CF (mol%) | 83 | 79 |
| Parameters | pH 7.0 | pH 6.0; NaAc Supplementation |
|---|---|---|
| Time period (h) | 0–232 | 232–380 |
| Vsyngas (NmL) | 1600 | 157 |
| rSmax (NmL/h) | 13.3 † 6.7 ‡ 3.2 * | 0.9 |
| OD (600nm) max | 1.71 | 0.65 |
| CDW (g/L) | 2.77 1 3.58 2 | 3.01 2 |
| Max. acetate produced (mM) | 51.1 | 38.9 |
| Max. acetate concentration (mM) | 51.1 | 138.3 |
| YP/S acetate (mmol/Lsyngas used) | 1.20 × 10−2 † 2.42 × 10−2 ‡ 2.16 × 10−3 * | 1.24 × 10−1 |
| Max. butyrate produced (mM) | 0.7 | 0.3 |
| Max. butyrate concentration (mM) | 0.7 | 0.7 |
| YP/S butyrate (mmol/Lsyngas used) | 3.29 × 10−4 † 1.03 × 10−3 ‡ 1.02 × 10−4 * | 1.26 × 10−3 |
| Parameters | pH 7.0 | pH 6.0; NaAc Supplementation |
|---|---|---|
| Time period (h) | 0–64 | 234–299 |
| CO consumed (mmol) | 14.4 | 0.4 |
| CO2 negative balance (mmol) | 7.1 | 1.9 |
| H2 consumed (mmol) | 11.2 | - |
| rCO (mmol/h) | 0.23 | 0.01 |
| rCO2 (mmol/h) | 0.11 | 0.03 |
| rH2 (mmol/h) | 0.18 | - |
| CF, total (mol%) | 88 | 48 |
| CF, partial (mol%) | CO: 100 CO2: 71 | CO: 93 CO2: 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.; Pinto, F.; Ortigueira, J.; Silva, C.; Gírio, F.; Moura, P. Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery. Energies 2021, 14, 7124. https://doi.org/10.3390/en14217124
Pacheco M, Pinto F, Ortigueira J, Silva C, Gírio F, Moura P. Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery. Energies. 2021; 14(21):7124. https://doi.org/10.3390/en14217124
Chicago/Turabian StylePacheco, Marta, Filomena Pinto, Joana Ortigueira, Carla Silva, Francisco Gírio, and Patrícia Moura. 2021. "Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery" Energies 14, no. 21: 7124. https://doi.org/10.3390/en14217124
APA StylePacheco, M., Pinto, F., Ortigueira, J., Silva, C., Gírio, F., & Moura, P. (2021). Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery. Energies, 14(21), 7124. https://doi.org/10.3390/en14217124