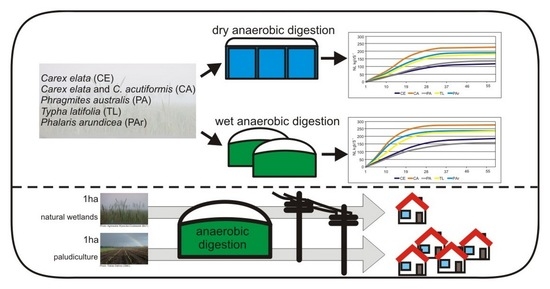
Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomethane Potential Test
2.1.1. Inoculum and Substrates
2.1.2. The Experimental Set-Up
Wet Fermentation
Dry Fermentation
2.1.3. Chemical Analyses
2.1.4. Biogas Calculations
2.2. Statistical Analysis
2.3. Energy Calculations
3. Results
3.1. Characteristics of the Inocula and Substrates
3.2. Methane Production
3.3. Energy Production from Wetland Biomass
4. Discussion
4.1. Chemical Composition of Wetland Plants
4.2. Biogas Yield
4.3. Energy Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| ADF | acid detergent fibre |
| ADL | acid detergent lignin |
| BMP | biomethane potential |
| CA | mixture (~50/50) of Carex elata and Carex acutiformis |
| CE | Carex elata |
| CF | crude fibre |
| CHP | combined heat and power |
| D–F | dry fermentation |
| DM | dry matter |
| GAX | glucuronoarabinoxylans |
| K | potassium (g kgTS−1) |
| Mtoe | million tonnes of oil equivalent |
| NDF | neutral detergent fibre |
| NL | normal litre, i.e., gas volume corrected to 0 °C and 1.013 bar |
| Nm3 | normal cubic metre |
| PA | Phragmites australis |
| PAr | Phalaris arundinacea |
| SBY | specific biogas yield (NL kgVS−1) |
| SMY | specific methane yield (NL kgVS−1) |
| T50 | the number of days required to reach 50% of the methane potential. |
| T95 | the number of days required to reach 95% of the methane potential. |
| TKN | total Kjeldahl nitrogen (g kgTS−1) |
| TL | Typha latifolia |
| TOC | total organic carbon (g kgTS−1) |
| TP | total phosphorus (g kgTS−1) |
| TS | total solids (%) |
| VS | volatile solids (% TS) |
| W–F | wet fermentation |
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group14 I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Narnaware Sunil, L.; Panwar, N.L. Biomass gasification for climate change mitigation and policy framework in India: A review. Bioresour. Technol. Rep. 2021, in press. [Google Scholar] [CrossRef]
- International Energy Agency. Outlook for Biogas and Biomethane 2020. Available online: https://iea.blob.core.windows.net/assets/03aeb10c-c38c-4d10-bcec-de92e9ab815f/Outlook_for_biogas_and_biomethane.pdf (accessed on 2 June 2021).
- Lüker-Jans, N.; Simmering, D.; Otte, A. The impact of biogas plants on regional dynamics of permanent grassland and maize area—The example of Hesse, Germany (2005–2010). Agric. Ecosyst. Environ. 2017, 241, 24–38. [Google Scholar] [CrossRef]
- Banaszuk, P.; Wysocka-Czubaszek, A.; Czubaszek, R.; Roj-Rojewski, S. Implications of biomass use for energy production (in Polish). Wieś i Rol. 2015, 169, 139–152. [Google Scholar]
- Kampman, B.; Leguijt, C.; Scholten, T.; Tallat-Kelpsaite, J.; Bruckmann, R.; Maroulis, G.; Lesschen, J.P.; Meesters, K.; Sikirica, N.; Elbersen, B. Optimal Use of Biogas from Waste Streams: An Assessment of the Potential of Biogas from Digestion in the EU beyond 2020; European Commission: Delft, The Netherlands, 2017; pp. 1–158. [Google Scholar]
- Nafziger, E. Cultivation and Environmental Impact of Corn. Oxford Research Encyclopedia of Environmental Science. 2019. Available online: https://oxfordre.com/environmentalscience/view/10.1093/acrefore/9780199389414.001.0001/acrefore-9780199389414-e-220 (accessed on 2 June 2021).
- Dobers, G.M. Acceptance of biogas plants taking into account space and place. Energy Policy 2019, 135, 110987. [Google Scholar] [CrossRef]
- Szajdak, L.W.; Jezierski, A.; Wegner, K.; Meysner, T.; Szczepański, M. Influence of drainage on peat organic matter: Implications for development, stability, and transformation. Molecules 2020, 25, 2587. [Google Scholar] [CrossRef] [PubMed]
- Tanneberger, F.; Appulo, L.; Ewert, S.; Lakner, S.; Brolcháin, N.Ó.; Peters, J.; Wichtmann, W. The Power of nature-based solutions: How peatlands can help us to achieve key EU sustainability objectives. Adv. Sustain. Syst. 2020, 5, 2000146. [Google Scholar] [CrossRef]
- Beer, F.; Wichtmann, W.; Villegas, L.; Agus, F. Paludiculture. In Recarbonizing Global Soils: A Technical Manual of Best Management Practices; FAO: Rome, Italy, 2021; Volume 5, pp. 179–189. [Google Scholar] [CrossRef]
- Tanneberger, F.; Wichtmann, W. Land use options for rewetted peatlands. In Carbon Credits from Peatland Rewetting. Climate-Biodiversity-Land Use, 1st ed.; Tanneberger, F., Wichtmann, W., Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2011; pp. 107–132. [Google Scholar]
- Bianchi, A.; Larmola, T.; Kekkonen, H.; Saarnio, S.; Lang, K. Review of greenhouse gas emissions from rewetted agricultural soils. Wetlands 2021, 41, 108. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; Oehmke, C.; Lambertini, C.; Eller, F.; Sorrell, B.K.; Mandiola, S.R.; Grootjans, A.P.; Brix, H.; Wichtmann, W.; Lamers, L.P.M.; et al. Nutrient removal potential and biomass production by Phragmites australis and Typha latifolia on European rewetted peat and mineral soils. Sci. Total Environ. 2020, 747, 141102. [Google Scholar] [CrossRef]
- Schröder, C.; Dahms, T.; Paulitz, J.; Wichtmann, W.; Wichmann, S. Towards large-scale paludiculture: Addressing the challenges of biomass harvesting in wet and rewetted peatlands. Mires Peat 2015, 16, 1–18. [Google Scholar]
- Tanneberger, F.; Kubacka, J. (Eds.) The Aquatic Warbler Conservation Handbook, 1st ed.; The Brandenburg State Office for Environment: Potsdam, Germany, 2018; pp. 1–260. [Google Scholar]
- Khiari, B.; Jeguirim, M.; Limousy, L.; Benneci, S. Biomass derived chars for energy applications. Renew. Sustain. Energy Rev. 2019, 108, 253–273. [Google Scholar] [CrossRef]
- Kandel, T.P.; Sutaryo, S.; Møller, H.B.; Jørgensen, U.; Lærke, P.E. Chemical composition and methane yield of reed canary grass as influenced by harvesting time and harvest frequency. Bioresour. Technol. 2013, 130, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Roj-Rojewski, S.; Wysocka-Czubaszek, A.; Czubaszek, R.; Kamocki, A.; Banaszuk, P. Anaerobic digestion of wetland biomass from conservation management for biogas production. Biomass Bioenerg. 2019, 122, 126–132. [Google Scholar] [CrossRef]
- Maucieri, C.; Camarotto, C.; Florio, G.; Albergo, R.; Ambric, A.; Trupo, M.; Borin, M. Bioethanol and biomethane potential production of thirteen pluri-annual herbaceous species. Ind. Crop. Prod. 2019, 129, 694–701. [Google Scholar] [CrossRef]
- Brix, H.; Sorrell, B.K.; Lorenzen, B. Are Phragmites-dominated wetlands a net source or net sink of greenhouse gases? Aquat. Bot. 2001, 69, 313–324. [Google Scholar] [CrossRef]
- Granéli, W. Common reed Phragmites australis (Cav.) Trin. Ex Steudel as energy source in Sweden. Int. J. Biomass Renew. 1984, 4, 183–206. [Google Scholar] [CrossRef]
- Kobbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Hartung, C.; Andrade, D.; Dandikas, V.; Eickenscheidt, T.; Drösler, M.; Zollfrank, C.; Heuwinkel, H. Suitability of paludiculture biomass as biogas substrate biogas yield and long-term effects on anaerobic digestion. Renew. Energy 2020, 159, 64–67. [Google Scholar] [CrossRef]
- Massé, D.; Gilbert, Y.; Savoie, P.; Bélanger, G.; Parent, G.; Babineau, D. Methane yield from switchgrass and reed canarygrass grown in Eastern Canada. Bioresour. Technol. 2011, 102, 10286–10292. [Google Scholar] [CrossRef] [PubMed]
- Hallam, A.; Anderson, I.C.; Buxton, D.R. Comparative economic analysis of perennial, annual, and intercrops for biomass production. Biomass Bioenerg. 2001, 21, 407–424. [Google Scholar] [CrossRef]
- Pociene, L.; Sarunaite, L.; Tivikiene, V.; Slepetys, J.; Kadziuliene, Z. The yield and composition of reed canary grass biomass as raw material for combustion. Biologija 2013, 59, 195–200. [Google Scholar] [CrossRef]
- Jasinskas, A.; Žaltauskas, A.; Kryževičienė, A. The investigation of growing and using of tall perennial grasses as energy crops. Biomass Bioenerg. 2008, 32, 981–987. [Google Scholar] [CrossRef]
- Stelmaszczyk, M.; Okruszko, T.; Meire, P. Nutrients availability and hydrological conditions of selected wetland ecosystems in the Biebrza river valley. Ann. Wars. Univ. Life Sci. SGGW Land Reclam. 2015, 47, 3–17. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P. GHG emissions and efficiency of Energy generation through anaerobic fermentation of wetland biomass. Energies 2020, 13, 6497. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, T.; Mei, Z.; Li, J.; Qiu, K.; Ge, Y. Dry anaerobic digestion technologies for agricultural straw and acceptability in China. Sustainability 2018, 10, 4588. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, E.M.; Samer, M.; Amer, M.A.; Amer, B.M.A. Biogas production using dry fermentation technology through co-digestion of manure and agricultural wastes. Environ. Dev. Sustain. 2021, 23, 8746–8757. [Google Scholar] [CrossRef]
- Stolze, Y.; Zakrzewski, M.; Maus, I.; Eikmeyer, F.; Jaenicke, S.; Rottmann, N.; Siebner, C.; Pühler, A.; Schlüter, A. Comparative metagenomics of biogas-producing microbial communities from production-scale biogas plants operating under wet or dry fermentation conditions. Biotechnol. Biofuels 2015, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, A.K.; Li, J.; Zhang, L.; Ban, Q.; Jin, Y. Comparison between wet and dry anaerobic digestions of cow dung under mesophilic and thermophilic conditions. Adv. Water Resour. Prot. 2013, 1, 28–38. [Google Scholar]
- Luning, L.; van Zundert, E.H.M.A.; Brinkmann, J.F. Comparison of dry and wet digestion for solid waste. Water Sci. Technol. 2013, 48, 15–20. [Google Scholar] [CrossRef]
- Vogel, T.; Ahlhaus, M.; Barz, M. Optimisation of biogas production from grass by dry-wet fermentation. In Proceedings of the 8th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 28–29 May 2009; pp. 21–26. [Google Scholar]
- Angelonidi, E.; Smith, S.R. A comparison of wet and dry anaerobic digestion processes for the treatment of municipal solid waste and food waste. Water Environ. J. 2015, 29, 549–557. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater; APHA: Washington, DC, USA, 1998; ISBN 9780875532356. [Google Scholar]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of biochemical methane potential and kinetics on the anaerobic digestion of vegetable crop residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Ciria, M.P.; Solano, M.L.; Soriano, P. Role of macrophyte Typha latifolia in a constructed wetland for wastewater treatment and assessment of its potential as a biomass fuel. Biosyst. Eng. 2005, 92, 535–544. [Google Scholar] [CrossRef]
- Grzelak, M.; Waliszewska, B.; Sieradzka, A.; Speak-Dźwigała, A. Ecological meadow communities with participation of species from sedge (Carex) family. J. Res. Appl. Agric. Eng. 2011, 56, 122–126. [Google Scholar]
- Kask, Ü.; Kask, L.; Link, S. Combustion characteristics of reed and its suitability as a boiler fuel. Mires Peat 2013, 13, 1–10. [Google Scholar]
- Platace, R.; Adamovics, A.; GulbeI, I. Evaluation of factors influencing calorific value of reed canary grass spring and autumn yield. In Proceedings of the Conference Engineering for rural development, Jelgava, Latvia, 23–24 May 2013; pp. 521–525. [Google Scholar]
- Waliszewska, B.; Janyszek, M.; Grzelak, M.; Gaweł, E. Characteristic of thermochemical parameters of aboveground parts of selected sedges (Carex L. Cyperaceae). IOSR J. Agric. Vet. Sci. 2013, 5, 1–6. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical characteristics of selected grass species from polish meadows and their potential utilization for energy generation purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Kakitis, A.; Ancans, D.; Nulle, I. Evaluation of combustion properties of biomass mixtures. In Proceedings of the Conference Engineering for Rural Development, Jelgava, Latvia, 29–30 May 2013; pp. 423–427. [Google Scholar]
- Murawski, M.; Grzelak, M.; Waliszewska, B.; Knioła, A.; Czekała, W. Energy value and yielding from extensively used meadows (in Polish). Fragm. Agron. 2015, 32, 71–78. [Google Scholar]
- Alberski, J.; Olszewska, M.; Bałuch-Małecka, A.; Kurzeja, M. Energy value of biomass from the extensively used Warkały-Trojan meadows (in Polish). Grass. Sci. Poland 2015, 18, 7–16. [Google Scholar]
- Roos, C.J. Biomass Drying and Dewatering for Clean Heat & Power, 1st ed.; U.U. Department of Enegy. CHP Technical Assistance Partnership: Olympia, WA, USA, 2009; p. 27.
- European Biomass Association. Statistical Report 2017; European Biomass Association: Brussels, Belgium, 2017. [Google Scholar]
- Niszczota, S.; Dziubiński, K.; Kupidura, A.; Miziołek, D.; Pacuszka, R.; Rafa, W.; Siestrzewitowska, A. Production of Agricultural and Horticultural Crops in 2019; Statistics Poland, Agriculture Department, Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2020. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/agricultural-and-horticultural-crops/production-of-agricultural-and-horticultural-crops-in-2019,2,4.html (accessed on 20 September 2021).
- Bauer, A.; Mayr, H.; Hopfner-Sixt, K.; Amon, T. Detailed monitoring of two biogas plants and mechanical solid–liquid separation of fermentation residues. J. Biotechnol. 2009, 142, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Rath, J. Biogas production from maize: Current state, challenges, and prospects. 1. Methane yield potential. Bioenerg. Res. 2012, 5, 1027–1042. [Google Scholar] [CrossRef]
- Joosten, H. The Global Peatland CO2 Picture. Peatland Status and Emissions in All Countries of the World; Wetlands International: Ede, The Netherlands, 2009; Available online: https://unfccc.int/sites/default/files/draftpeatlandco2report.pdf (accessed on 28 October 2021).
- Tanneberger, F.; Moen, A.; Barthelmes, A.; Lewis, E.; Miles, L.; Sirin, A.; Tegetmeyer, C.; Joosten, H. Mires in Europe—Regional Diversity, Condition and Protection. Diversity 2021, 13, 381. [Google Scholar] [CrossRef]
- Gunther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.D.; Lupascu, M.; Wijedasa, L.S. Paludiculture as a sustainable land use alternative for tropical peatlands: A review. Sci. Total Environ. 2021, 753, 142111. [Google Scholar] [CrossRef]
- Joosten, H.; Tapio-Bistrom, M.-L.; Tol, S. (Eds.) Peatlands—Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use, 2nd ed.; Food and Agriculture Organization of the United Nations and Wetlands International: Rome, Italy; Ede, The Netherlands, 2012; p. 114. [Google Scholar]
- IPCC. Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014; p. 354. [Google Scholar]
- Biancalani, R.; Avagyan, A. (Eds.) Towards climate-responsible peatlands management. In Mitigation of Climate Change in Agriculture Series 9; FAO: Rome, Italy, 2014. [Google Scholar]
- Parzych, A.E.; Sobisz, Z.; Jonczak, J. Comparing Carex species of mid-forest spring ecosystems in terms of ability to accumulate macro- and microelements. J. Ecol. Eng. 2017, 18, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Baran, M.; Váradyová, Z.; Kráâmar, S.; Hedbávný, J. The common reed (Phragmites australis) as a source of roughage in ruminant nutrition. Acta Vet. Brno 2002, 71, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Oleszek, M.; Król, A.; Tys, J.; Matyka, M.; Kulik, M. Comparison of biogas production from wild and cultivated varieties of reed canary grass. Bioresour. Technol. 2014, 156, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Ostrowska, A.; Porębska, G. Chemical Composition of Plants, Its Interpretation and Use in Environmental Protection, 1st ed.; Instytut Ochrony Środowiska: Warszawa, Poland, 2002; p. 165. [Google Scholar]
- Lopez-Gonzalez, D.; Avalos-Ramirez, A.; Giroir-Fendler, A.; Godbout, S.; Fernandez-Lopez, M.; Sanchez-Silva, L.; Valverde, J.L. Combustion kinetic study of woody and herbaceous crops by thermal analysis coupled to mass spectrometry. Energy 2015, 90, 1626–1635. [Google Scholar] [CrossRef]
- Florio, G. Agronomic and Environmental Evaluation of Perennial Herbaceous Plants Fertilized with Slurry to Obtain Biomass for Bioenergy. Ph.D. Thesis, Università degli Studi di Padova, Padova, Italy, 31 January 2014. [Google Scholar]
- Borin, M.; Florio, G.; Barber, A.; Cirelli, G.L.; Albergo, R.; Palazzo, S. Preliminary evaluation of macrophyte wetland biomasses to obtain second generation ethanol. In Proceedings of the 19th European Biomass Conference and Exhibition, Berlin, Germany, 6–10 June 2011. [Google Scholar]
- Wöhler-Geske, A.; Moschner, C.R.; Gellerich, A.; Militz, H.; Greef, J.-M.; Hartung, E. Provenances and properties of thatching reed (Phragmites australis). Appl. Agric. For. Res. 2016, 66, 1–10. [Google Scholar] [CrossRef]
- Ohlsson, L.-O.; Karlsson, S.; Rupar-Gadd, K.; Albers, E.; Welander, U. Evaluation of Laminaria digitata and Phragmites australis for biogas production and nutrient recycling. Biomass Bioenerg. 2020, 140, 105670. [Google Scholar] [CrossRef]
- Van Tran, G.; Unpaprom, Y.; Ramaraj, R. Methane productivity evaluation of an invasive wetland plant, common reed. Biomass Convers. Biorefin. 2020, 10, 689–695. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T.; Hao, X. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol. 2015, 185, 79–88. [Google Scholar] [CrossRef]
- Melts, I.; Normak, A.; Nurk, L.; Heinsoo, K. Chemical characteristics of biomass from nature conservation management for methane production. Bioresour. Technol. 2014, 167, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Melts, I.; Ivask, M.; Geetha, M.; Takeuchi, K.; Heinsoo, K. Combining bioenergy and nature conservation: An example in wetlands. Renew. Sustain. Energy Rev. 2019, 111, 293–302. [Google Scholar] [CrossRef]
- Stypiński, P.; Chodkiewicz, A. The fodder value of plant communities grazed by Koniks in the Biebrza National Park (in Polish). Zesz. Nauk. 2011, 47, 222–230. [Google Scholar]
- Smith, B.G.; Harris, P.J. The polysaccharide composition of Poales cell walls: Poaceae cell walls are not unique. Biochem. Syst. Ecol. 1999, 27, 33–53. [Google Scholar] [CrossRef]
- Vogel, J. Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 2008, 11, 301–307. [Google Scholar] [CrossRef]
- Elhaak, M.A.; Mohsen, A.A.; Hamada, E.A.M.; El-Gebaly, F.E. Biofuel production from Phragmites australis (Cav.) and Typha domingensis (Pers.) Plants of Burullus Lake. Egypt. J. Exp. Biol. 2015, 11, 237–243. [Google Scholar]
- Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable ethanol production from common reed (Phragmites australis) through simultaneuos saccharification and fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef] [Green Version]
- Honoré, M.; Pimbert, S.; Lecompte, T. Characterisation of plant flours for biocomposite applications focussing on Phragmites australis properties. Biosyst. Eng. 2020, 197, 367–377. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Yu, H.-Q. Anaerobic digestion of cattail by rumen cultures. Waste Manag. 2006, 26, 1222–1228. [Google Scholar] [CrossRef]
- Aysu, T.; Turhan, M.; Küçük, M.M. Liquefaction of Typha latifolia by supercritical fluid extraction. Bioresour. Technol. 2012, 107, 464–470. [Google Scholar] [CrossRef]
- Sopajarn, A.; Sangwichien, C. Optimization of enzymatic saccharification of alkali pretreated Typha angustifolia for glucose production. Int. J. Chem. Eng. Appl. 2015, 6, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Mishra, C.; Behera, S.S.; Thatoi, H. Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass—A review. Renew. Sustain. Energy Rev. 2017, 78, 1007–1032. [Google Scholar] [CrossRef]
- Robbins, M.P.; Evans, G.; Valentine, J.; Donnison, I.S.; Allison, G.G. New opportunities for the exploitation of energy crops by thermochemical conversion in Northern Europe and the UK. Prog. Energy Combust. Sci. 2012, 38, 138–155. [Google Scholar] [CrossRef]
- Grzelak, M. Biomass fodder value of high-sedge rushes from ecological grasslands. J. Res. Appl. Agric. Eng. 2010, 55, 95–98. [Google Scholar]
- Reza, M.T.; Becker, W.; Sachsenheimer, K.; Mumme, J. Hydrothermal carbonization (HTC): Near infrared spectroscopy and partial least-squares regression for determination of selective components in HTC solid and liquid products derived from maize silage. Bioresour. Technol. 2014, 161, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Swiontek Brzezinska, M. The quantity and quality of methanogenic microorganisms based on rRNA analysis and their relation to methane production. Afr. J. Microbiol. Res. 2013, 7, 3389–3395. [Google Scholar] [CrossRef] [Green Version]
- Ribas, T.M.B.; Neumann, M.; de Souza, A.M.; Dochwat, A.; de Almeida, E.R.; Horst, E.H. Effect of inoculants combining Lactobacillus buchneri strain LN40177 in different strata of the silo. Acta Sci.-Anim. Sci. 2019, 41, e44847. [Google Scholar] [CrossRef] [Green Version]
- Melts, I.; Heinsoo, K. Seasonal dynamics of bioenergy characteristics in grassland functional groups. Grass Forage Sci. 2015, 70, 571–581. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from landscape management of grassland used for biogas production: Effects of harvest date and silage additives on feedstock quality and methane yield. Grass Forage Sci. 2014, 69, 549–566. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Triolo, J.M.; Pedersen, L.; Qu, H.; Sommer, S.G. Biochemical methane potential and anaerobic biodegradability of non-herbaceous and herbaceous phytomass in biogas production. Bioresour. Technol. 2012, 125, 226–232. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, G.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [Green Version]
- Dragoni, F.; Giannini, V.; Ragaglini, G.; Bonari, E.; Silvestri, N. Effect of harvest time and frequency on biomass quality and biomethane potential of common reed (Phragmites australis) under paludiculture conditions. Bioenerg. Res. 2017, 10, 1066–1078. [Google Scholar] [CrossRef]
- Komulainen, M.; Simi, P.; Hagelberg, E.; Ikonen, I.; Lyytynen, S. Reed energy: Possibilities of using the common reed for energy generation in Southern Finland. Rep. Turku Univ. Appl. Sci. 2008, 67, 5–75. [Google Scholar]
- Lizasoain, J.; Rincon, M.; Theuretzbacher, F.; Enguídanos, R.J.; Nielsen, P.J.; Potthast, A.; Zweckmair, T.; Gronauer, A.; Bauer, A. Biogas production from reed biomass: Effect of pretreatment using different steam explosion conditions. Biomass Bioenerg. 2016, 95, 84–91. [Google Scholar] [CrossRef]
- Eller, F.; Ehde, P.P.; Oehmke, C.; Ren, L.; Brix, H.; Sorrell, B.K.; Weisner, S.E.B. Biomethane Yield from different European Phragmites australis genotypes, compared with other herbaceous wetland species grown at different fertilization regimes. Resources 2020, 9, 57. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Blasius, J.P.; Contrera, R.C.; Maintinguer, S.I.; de Castro, M.C.A.A. Effects of temperature, proportion and organic loading rate on the performance of anaerobic digestion of food waste. Biotechnol. Rep. 2020, 27, e00503. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.H.; Kotova, P.; Shaw, C.; Hong, Y.; Chang, S. Impacts of temperature and solids retention time, and possible mechanisms of biological hydrolysis pretreatment on anaerobic digestion. Water 2020, 12, 3166. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Huttunen, S.; Lehtinen, T.M.; Rintala, J.A. Anaerobic digestion of grass silage in batch leach bed processes for methane production. Bioresour. Technol. 2008, 99, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Walkowska, K. Energy Consumption in Households in 2018; Statistics Poland, Enterprises Department, Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2019. Available online: https://stat.gov.pl/en/topics/environment-energy/energy/energy-consumption-in-households-in-2018,2,5.html (accessed on 7 October 2021).







| Inoculum for Wet Fermentation | Inoculum for Dry Fermentation | Carex elata (CE) | Carex acutiformis + Carex elata (CA) | Phragmites australis (PA) | Typha latifolia (TL) | Phalaris Arundinacea (PAr) | |
|---|---|---|---|---|---|---|---|
| Total solids (TS), % | 5.06 ± 0.04 A | 11.91 ± 0.15 B | 45.94 ± 0.12 a | 25.89 ± 0.36 b | 44.76 ± 0.90 a | 25.25 ± 0.33 b | 34.250 ± 48 c |
| Volatile solids (VS), % TS | 78.10 ± 0.07 A | 83.11 ± 0.31 B | 95.50 ± 0.25 a | 92.38 ± 0.22 b | 91.82 ± 0.19 c | 93.65 ± 0.16 d | 93.17 ± 0.08 d |
| pH | 7.99 ± 0.02 | n.a. | 5.32 ± 0.02 a | 5.56 ± 0.08 b | 5.72 ± 0.03 c | 5.07 ± 0.02 d | 4.76 ± 0.02 e |
| Total Kjeldahl nitrogen (TKN), g kgTS−1 | 90.53 ± 3.18 A | 46.65 ± 0.13 B | 16.94 ± 0.31 a | 23.73 ± 0.52 b | 21.21 ± 0.97 c | 16.68 ± 0.18 a | 25.04 ± 1.49 b |
| Total phosphorus (TP), g kgTS−1 | 9.46 ± 0.19 A | 9.62 ± 0.92 A | 0.82 ± 0.05 a | 2.72 ± 0.08 b | 1.30 ± 0.10 c | 1.95 ± 0.06 d | 2.35 ± 0.04 e |
| Total potassium (K), g kgTS−1 | 54.73 ± 1.67 A | 25.85 ± 0.77 B | 5.32 ± 0.19 a | 16.04 ± 0.35 b | 4.22 ± 0.28 c | 8.91 ± 0.21 d | 11.02 ± 0.13 e |
| Total organic carbon (TOC), g kgTS−1 | 409.81 ± 5.41 A | 442.96 ± 4.01 B | 469.54 ± 16.37 a | 465.16 ± 2.60 a | 452.50 ± 12.36 a | 454.97 ± 2.51 a | 459.06 ± 13.58 a |
| Carex elata (CE) | Carex acutiformis + Carex elata (CA) | Phragmites australis (PA) | Typha latifolia (TL) | Phalaris arundinacea (PAr) | |
|---|---|---|---|---|---|
| Crude fibre, % TS | 32.43 ± 1.46 a | 37.62 ± 0.89 b | 36.80 ± 3.71 ab | 44.06 ± 0.23 c | 36.39 ± 1.29 ab |
| Lignin, % TS | 20.79 ± 2.82 a | 16.03 ± 1.54 b | 21.90 ± 1.77 a | 36.40 ± 1.04 c | 22.19 ± 0.78 a |
| Hemicellulose, % TS | 25.16 ± 0.75 a | 27.89 ± 0.53 a | 27.77 ± 1.09 a | 19.22 ± 1.22 b | 37.55 ± 1.04 c |
| Cellulose, % TS | 26.76 ± 2.66 a | 29.37 ± 0.87 a | 25.32 ± 1.48 a | 25.69 ± 2.15 a | 16.90 ± 1.29 b |
| Lignification | 0.7 | 0.5 | 0.9 | 1.4 | 1.3 |
| Plant Species | Type of Fermentation | |
|---|---|---|
| Wet | Dry | |
| Carex elata (CE) | 190 ± 5 aA | 121 ± 5 aB |
| Carex acutiformis + Carex elata (CA) | 275 ± 3 bA | 228 ± 1 bB |
| Phragmites australis (PA) | 160 ± 28 cA | 138 ± 3 cA |
| Typha latifolia (TL) | 237 ± 13 dA | 185 ± 6 dB |
| Phalaris arundinacea (PAr) | 238 ± 2 dA | 189 ± 3 dB |
| Maize silage [53] | 195–581 | – |
| Plant Species | Electricity | Heat | ||||||
|---|---|---|---|---|---|---|---|---|
| kWh ha−1 | kWh tDM−1 | GJ ha−1 | GJ tDM−1 | |||||
| W–F | D–F | W–F | D–F | W–F | D–F | W–F | D–F | |
| Carex elata (CE) | 3569 | 2472 | 528 | 365 | 11.18 | 9.38 | 1.65 | 1.39 |
| Carex acutiformis + Carex elata (CA) | 1592 | 1439 | 748 | 675 | 4.99 | 5.46 | 2.34 | 2.56 |
| Phragmites australis (PA) | 3708 | 3471 | 429 | 402 | 11.62 | 13.17 | 1.35 | 1.53 |
| Typha latifolia (TL) | 3157 | 2682 | 628 | 534 | 9.89 | 10.18 | 1.97 | 2.03 |
| Phalaris arundinacea (PAr) | 3581 | 3103 | 641 | 555 | 11.22 | 11.77 | 2.01 | 2.11 |
| Maize | 13,622 | – | 973 | – | 42.69 | – | 3.05 | – |
| Plant Species | Electricity Production from AD | Heat Production from AD | ||
|---|---|---|---|---|
| W–F | D–F | W–F | D–F | |
| kWh ha−1 | GJ ha−1 | |||
| Carex elata (CE) | 6332 | 4386 | 19.84 | 16.64 |
| Carex acutiformis + Carex elata (CA) | 5981 | 5403 | 18.74 | 20.50 |
| Phragmites australis (PA) | 6870 | 6431 | 21.52 | 24.40 |
| Typha latifolia (TL) | 12,567 | 10,679 | 39.37 | 40.52 |
| Phalaris arundinacea (PAr) | 9610 | 8326 | 30.10 | 31.60 |
| Maize | 13,622 | – | 42.69 | – |
| Plant Species | Total Kjeldahl Nitrogen (TKN) | Total Phosphorus (TP) | Total Potassium (K) | Total Organic carbon (TOC) | Reference |
|---|---|---|---|---|---|
| g kg−1 | |||||
| Carex elata | 15.3 | 1.03 | – | 447 | [19] |
| 10.4 | – | – | 444.6 | [67] | |
| Carex acutiformis + Carex elata | – | – | 21.36 | 431 | [66] |
| 14.8 | 2.2 | 23.0 | – | [61] | |
| Phragmites australis | – | 1.57 | 10.90 | 870.5 | [62] |
| 6.27 | 0.625 | 2.114 | 392 | [65] | |
| 23.7 | 1.73 | – | 402 | [19] | |
| 13.4 | – | – | 443.4 | [67] | |
| 0.53–6.68 | 0.04–0.34 | 0.2–8.0 | 473–526.3 | [68] | |
| 13.7 | 2.2 | – | – | [69] | |
| 11.4 | – | – | 506.8 | [70] | |
| Typha latifolia | 7 | – | – | 449 | [71] |
| Phalaris arundinacea | 9.74/11.3 | – | – | 444/452 | [27] |
| 6.53 | 11.371 | 6.354 | 437 | [65] | |
| 14.6 ± 1.2 | 2.52 ± 0.06 | 5.83 ± 0.14 | 619.4 ± 12.5 | [63] | |
| 37.7 | 5.53 | – | 403 | [19] | |
| – | – | 17.93 | 422 | [66] | |
| 15.2 | – | – | 421.6 | [67] | |
| Sedges | 15.70 | 1.39 | 6.29 | [72] | |
| 15.2 | – | – | 421.6 | [67] | |
| 15 | 1.2 | 10.7 | 448 | [73] | |
| 13–31 | 1–4 | 5–12 | – | [64] | |
| 10–14.8 | 1.9–2.2 | 18.3–25.1 | – | [61] | |
| – | 1.3 | 10.7 | – | [74] | |
| Plant Species | Cellulose | Hemicellulose | Lignin | Reference |
|---|---|---|---|---|
| % TS | ||||
| Carex elata | 33.3 | 34.6 | 11.1 | [67] |
| Carex acutiformis + Carex elata | 37.4 | 17.2 | 20.3 | [44,47] |
| 29.7 | 36.0 | 7.9 | [66] | |
| Phragmites australis | 48.3 (summer) | 20.1 | 15.3 | [77] |
| 41.7 (winter) | 19.4 | 21.0 | ||
| 39.3 | 27.2 | 23.6 | [47] | |
| 35.1 | 30.2 | 22.0 | [45] | |
| 26.2 | 33.3 | 7.3 | [67] | |
| 38.1 | 20.5 | 23.0 | [78] | |
| 38.8–57.5 | 20.9–40.2 | 8.4–17.2 | [68] | |
| 41.2 | 30.9 | 11.8 | [79] | |
| 32.0 | 29.0 | 18.6 | [70] | |
| Typha latifolia | 32.6 | 22.1 | 5.4 | [24] |
| 20.8 | 22.6 | 10.5 | [80] | |
| 37.3 | 32.8 | 21.7 | [81] | |
| 45.3 | 19.8 | 8.8 | [71] | |
| 38.5 | 37.6 | 12.8 | [82] | |
| Phalaris arundinacea | 29.8 | 25.8 | 8.0 | [63] |
| 32.6 | 19.8 | 24.6 | [47] | |
| 38–45 | 20–25 | 18–21 | [83] | |
| 38.7 | 31.7 | 15.4 | [45] | |
| 29.5 | 22.8 | 8.9 | [73] | |
| 28.7 | 33.8 | 5.7 | [67] | |
| 28.0 | 22.0 | 14.0 | [84] | |
| 27.6 | 27.5 | 2.9 | [24] | |
| Sedges | 34.86–36.75 | 18.34 | 17.56–25.18 | [41,85] |
| 30.1–46.2 | 9.8–28.5 | 11.9–28.1 | [44,47] | |
| 30.22 | 32.39 | 5.06 | [72] | |
| 29.7 | 30.8 | 5.6 | [73] | |
| Maize | 32.7 | 26.3 | 7.0 | [86] |
| 18.39 | 19.59 | 1.43 | [87] | |
| 24.09 | 15.58 | 5.77 | [88] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Banaszuk, P. Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies 2021, 14, 8373. https://doi.org/10.3390/en14248373
Czubaszek R, Wysocka-Czubaszek A, Wichtmann W, Banaszuk P. Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies. 2021; 14(24):8373. https://doi.org/10.3390/en14248373
Chicago/Turabian StyleCzubaszek, Robert, Agnieszka Wysocka-Czubaszek, Wendelin Wichtmann, and Piotr Banaszuk. 2021. "Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies" Energies 14, no. 24: 8373. https://doi.org/10.3390/en14248373
APA StyleCzubaszek, R., Wysocka-Czubaszek, A., Wichtmann, W., & Banaszuk, P. (2021). Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies, 14(24), 8373. https://doi.org/10.3390/en14248373