High Purity/Recovery Separation of Propylene from Propyne Using Anion Pillared Metal-Organic Framework: Application of Vacuum Swing Adsorption (VSA)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Adsorption of Pure Compounds on SIFSIX-3-Ni
3.2. Breakthrough Adsorption
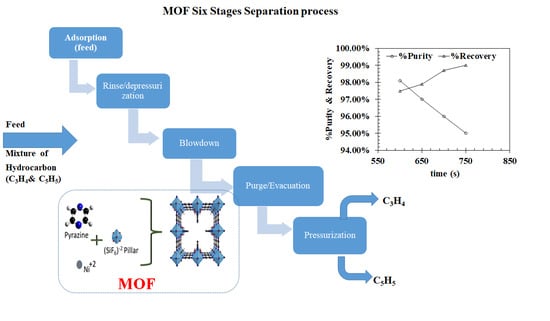
3.3. Vacuum Pressure Adsorption (VPA)
3.4. Energy Consumption, Purity, Recovery and Productivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashok, A.; Kumar, A.; Bhosale, R.; Saad, M.A.S.; AlMomani, F.; Tarlochan, F. Study of ethanol dehydrogenation reaction mechanism for hydrogen production on combustion synthesized cobalt catalyst. Int. J. Hydrogen Energy 2017, 42, 23464–23473. [Google Scholar] [CrossRef]
- Mundstock, A.; Wang, N.; Friebe, S.; Caro, J. Propane/propene permeation through Na-X membranes: The interplay of separation performance and pre-synthetic support functionalization. Microporous Mesoporous Mater. 2015, 215, 20–28. [Google Scholar] [CrossRef]
- Martins, V.F.; Seabra, R.; Silva, P.; Ribeiro, A.M.; Cho, K.H.; Lee, U.-H.; Chang, J.-S.; Loureiro, J.M.; Rodrigues, A.E.; Ferreira, A. C2/C3 Hydrocarbon Separation by Pressure Swing Adsorption on MIL-100 (Fe). Ind. Eng. Chem. Res. 2020, 59, 10568–10582. [Google Scholar] [CrossRef]
- Lan, T.; Li, L.; Chen, Y.; Wang, X.; Yang, J.; Li, J. Opportunities and critical factors of porous metal–organic frameworks for industrial light olefins separation. Mater. Chem. Front. 2020, 4, 1954–1984. [Google Scholar] [CrossRef]
- Yang, R.; Gao, R.; Qian, Z.; Wang, Y. Batch and fixed bed column selective adsorption of C6, C8 and C10 linear α-olefins from binary liquid olefin/paraffin mixtures onto 5A and 13X microporous molecular sieves. Sep. Purif. Technol. 2020, 230, 115884. [Google Scholar] [CrossRef]
- Andrade, M.; Relvas, F.; Mendes, A. Highly propylene equilibrium selective carbon molecular sieve adsorbent. Sep. Purif. Technol. 2020, 245, 116853. [Google Scholar] [CrossRef]
- Tran, N.T.; Kim, J.; Othman, M.R. Microporous ZIF-8 and ZIF-67 membranes grown on mesoporous alumina substrate for selective propylene transport. Sep. Purif. Technol. 2020, 233, 116026. [Google Scholar] [CrossRef]
- Li, L.; Guo, L.; Zheng, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Calcium-Based Metal–Organic Framework for Simultaneous Capture of Trace Propyne and Propadiene from Propylene. ACS Appl. Mater. Interfaces 2020, 12, 17147–17154. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Krishna, R.; Wang, Y.; Yang, J.; Wang, X.; Li, J. Exploiting the gate opening effect in a flexible MOF for selective adsorption of propyne from C1/C2/C3 hydrocarbons. J. Mater. Chem. A 2016, 4, 751–755. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Zhang, Y.; Yang, Q.; Xing, H. A highly sensitive flexible metal–organic framework sets a new benchmark for separating propyne from propylene. J. Mater. Chem. A 2018, 6, 24452–24458. [Google Scholar] [CrossRef]
- Peng, Y.L.; He, C.; Pham, T.; Wang, T.; Li, P.; Krishna, R.; Forrest, K.A.; Hogan, A.; Suepaul, S.; Space, B. Robust microporous metal–organic frameworks for highly efficient and simultaneous removal of propyne and propadiene from propylene. Angew. Chemie Int. Ed. 2019, 58, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, X.; Yang, Q.; Qian, S.; Wu, H.; Bao, Z.; Zhang, Z.; Ren, Q.; Zhou, W.; Chen, B. A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials. Adv. Mater. 2018, 30, 1705374. [Google Scholar] [CrossRef] [PubMed]
- Abedini, H.; Shariati, A.; Khosravi-Nikou, M.R. Adsorption of propane and propylene on M-MOF-74 (M=Cu, Co): Equilibrium and kinetic study. Chem. Eng. Res. Des. 2020, 153, 96–106. [Google Scholar] [CrossRef]
- Da Silva, F.A.; Rodrigues, A.E. Adsorption equilibria and kinetics for propylene and propane over 13X and 4A zeolite pellets. Ind. Eng. Chem. Res. 1999, 38, 2051–2057. [Google Scholar] [CrossRef]
- Jorge, M.; Lamia, N.; Rodrigues, A.E. Molecular simulation of propane/propylene separation on the metal–organic framework CuBTC. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 357, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Lamia, N.; Wolff, L.; Leflaive, P.; Sá Gomes, P.; Grande, C.A.; Rodrigues, A.E. Propane/propylene separation by simulated moving bed I. Adsorption of propane, propylene and isobutane in pellets of 13X zeolite. Sep. Sci. Technol. 2007, 42, 2539–2566. [Google Scholar] [CrossRef]
- Martins, V.F.D.; Ribeiro, A.M.; Plaza, M.G.; Santos, J.C.; Loureiro, J.M.; Ferreira, A.F.P.; Rodrigues, A.E. Gas-phase simulated moving bed: Propane/propylene separation on 13X zeolite. J. Chromatogr. A 2015, 1423, 136–148. [Google Scholar] [CrossRef]
- Plaza, M.G.; Ribeiro, A.M.; Ferreira, A.; Santos, J.C.; Lee, U.H.; Chang, J.-S.; Loureiro, J.M.; Rodrigues, A.E. Propylene/propane separation by vacuum swing adsorption using Cu-BTC spheres. Sep. Purif. Technol. 2012, 90, 109–119. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Tang, Y.; Wen, Y.; Lv, Z.; Liu, S.; Li, X.; Zhou, X. Propane-selective design of zirconium-based MOFs for propylene purification. Chem. Eng. Sci. 2020, 219, 115604. [Google Scholar] [CrossRef]
- Das, M.C.; Guo, Q.; He, Y.; Kim, J.; Zhao, C.-G.; Hong, K.; Xiang, S.; Zhang, Z.; Thomas, K.M.; Krishna, R. Interplay of metalloligand and organic ligand to tune micropores within isostructural mixed-metal organic frameworks (M’ MOFs) for their highly selective separation of chiral and achiral small molecules. J. Am. Chem. Soc. 2012, 134, 8703–8710. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Krishna, R.; Wang, X.; Li, B.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Flexible–robust metal–organic framework for efficient removal of propyne from propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, H.M.; He, C.; Lin, R.B.; Krishna, R.; Wu, H.; Zhou, W.; Li, J.; Li, B.; Chen, B. A metal–organic framework with suitable pore size and specific functional sites for the removal of trace propyne from propylene. Angew. Chemie 2018, 130, 15403–15408. [Google Scholar] [CrossRef]
- Wang, X.; Krishna, R.; Li, L.; Wang, B.; He, T.; Zhang, Y.-Z.; Li, J.-R.; Li, J. Guest-dependent pressure induced gate-opening effect enables effective separation of propene and propane in a flexible MOF. Chem. Eng. J. 2018, 346, 489–496. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in Metal–Organic Frameworks: A fundamental understanding. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J. Chromatogr. A 2017, 1530, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Grande, C.A.; Gascon, J.; Kapteijn, F.; Rodrigues, A.E. Propane/propylene separation with Li-exchanged zeolite 13X. Chem. Eng. J. 2010, 160, 207–214. [Google Scholar] [CrossRef]
- Silva, F.A.D.; Rodrigues, A.E. Propylene/propane separation by vacuum swing adsorption using 13X zeolite. AIChE J. 2001, 47, 341–357. [Google Scholar] [CrossRef]
- Madden, D.; Albadarin, A.B.; O’Nolan, D.; Cronin, P.; Perry IV, J.J.; Solomon, S.; Curtin, T.; Khraisheh, M.; Zaworotko, M.J.; Walker, G.M. Metal-Organic Material Polymer Coatings for Enhanced Gas Sorption Performance and Hydrolytic Stability Under Humid Conditions. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zaworotko, M.J. Crystal Engineering of Hybrid Coordination Networks: From Form to Function. Trends Chem. 2020, 2, 506–518. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y.; Liao, S.; Ke, Q.; Qiao, Z.; Wei, Y. Adsorption and separation of propane/propylene on various ZIF-8 polymorphs: Insights from GCMC simulations and the ideal adsorbed solution theory (IAST). Chem. Eng. J. 2020, 386, 123945. [Google Scholar] [CrossRef]
- Dhoke, C.; Cloete, S.; Krishnamurthy, S.; Seo, H.; Luz, I.; Soukri, M.; Park, Y.-k.; Blom, R.; Amini, S.; Zaabout, A. Sorbents screening for post-combustion CO2 capture via combined temperature and pressure swing adsorption. Chem. Eng. J. 2020, 380, 122201. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Qasem, N.A.A.; Antar, M.A. Carbon dioxide adsorption separation from dry and humid CO2/N2 mixture. Comput. Chem. Eng. 2018, 117, 221–235. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Bamidele, O.; Habib, M. Evaluation of Mg-MOF-74 for post-combustion carbon dioxide capture through pressure swing adsorption. Int. J. Energy Res. 2015, 39, 1994–2007. [Google Scholar] [CrossRef]
- Karra, J.R.; Grabicka, B.E.; Huang, Y.-G.; Walton, K.S. Adsorption study of CO2, CH4, N2, and H2O on an interwoven copper carboxylate metal–organic framework (MOF-14). J. Colloid Interface Sci. 2013, 392, 331–336. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Kumar, V.; Giannakoudakis, D.A.; Boukhvalov, D.W. Adsorptive removal of an eight-component volatile organic compound mixture by Cu-, Co-, and Zr-metal-organic frameworks: Experimental and theoretical studies. Chem. Eng. J. 2020, 397, 125391. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Li, W.; Huang, X.; Luan, S.; Hou, X.; Zhang, M.; Wang, Q. CO2 mediated fabrication of hierarchically porous metal-organic frameworks. Microporous Mesoporous Mater. 2019, 277, 154–162. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energy 2018, 210, 317–326. [Google Scholar] [CrossRef]
- Bahamon, D.; Díaz-Márquez, A.; Gamallo, P.; Vega, L.F. Energetic evaluation of swing adsorption processes for CO2 capture in selected MOFs and zeolites: Effect of impurities. Chem. Eng. J. 2018, 342, 458–473. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Hoffmann, F.; Fröba, M. Molecular simulation of hydrogen adsorption in metal-organic frameworks. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 357, 35–42. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Xia, Y.A.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb (II). Desalination 2018, 430, 120–127. [Google Scholar] [CrossRef]
- Kumar, A.; Hua, C.; Madden, D.G.; O’Nolan, D.; Chen, K.-J.; Keane, L.-A.J.; Perry, J.J.; Zaworotko, M.J. Hybrid ultramicroporous materials (HUMs) with enhanced stability and trace carbon capture performance. Chem. Commun. 2017, 53, 5946–5949. [Google Scholar] [CrossRef] [Green Version]
- Khraisheh, M.; Mukherjee, S.; Kumar, A.; Al Momani, F.; Walker, G.; Zaworotko, M.J. An overview on trace CO2 removal by advanced physisorbent materials. J. Environ. Manag. 2020, 255, 109874. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-T.; Liu, Q.-Y.; Yang, L.; He, C.-T.; Li, L.; Wang, Y.-L. Fluorinated Biphenyldicarboxylate-Based Metal–Organic Framework Exhibiting Efficient Propyne/Propylene Separation. Inorganic Chem. 2020, 59, 4030–4036. [Google Scholar] [CrossRef]
- Wen, H.-M.; Li, L.; Lin, R.-B.; Li, B.; Hu, B.; Zhou, W.; Hu, J.; Chen, B. Fine-tuning of nano-traps in a stable metal–organic framework for highly efficient removal of propyne from propylene. J. Mater. Chem. A 2018, 6, 6931–6937. [Google Scholar] [CrossRef]
- Park, J.; Lively, R.P.; Sholl, D.S. Establishing upper bounds on CO2 swing capacity in sub-ambient pressure swing adsorption via molecular simulation of metal–organic frameworks. J. Mater. Chem. A 2017, 5, 12258–12265. [Google Scholar] [CrossRef]
- Guang, C.; Zhao, X.; Zhang, Z.; Gao, J.; Li, M. Optimal design and performance enhancement of heteroazeotropic and pressure-swing coupling distillation for downstream isopropanol separation. Sep. Purif. Technol. 2020, 242, 116836. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Campo, M.C.; Narin, G.; Santos, J.C.; Ferreira, A.; Chang, J.-S.; Hwang, Y.K.; Seo, Y.-K.; Lee, U.-H.; Loureiro, J.M. Pressure swing adsorption process for the separation of nitrogen and propylene with a MOF adsorbent MIL-100 (Fe). Sep. Purif. Technol. 2013, 110, 101–111. [Google Scholar] [CrossRef]
- Da Silva, F.A.; Rodrigues, A.E. Propylene/propane separation by pressure swing adsorption. In Adsorption Science and Technology; World Scientific: Singapore, 2000; pp. 537–541. [Google Scholar]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Dasgupta, S.; Biswas, N.; Gode, N.G.; Divekar, S.; Nanoti, A.; Goswami, A.N. CO2 recovery from mixtures with nitrogen in a vacuum swing adsorber using metal organic framework adsorbent: A comparative study. Int. J. Greenh. Gas Control 2012, 7, 225–229. [Google Scholar] [CrossRef]
- Pai, K.N.; Baboolal, J.D.; Sharp, D.A.; Rajendran, A. Evaluation of diamine-appended metal-organic frameworks for post-combustion CO2 capture by vacuum swing adsorption. Sep. Purif. Technol. 2019, 211, 540–550. [Google Scholar] [CrossRef]
- Mofarahi, M.; Sadrameli, M.; Towfighi, J. Four-bed vacuum pressure swing adsorption process for propylene/propane separation. Ind. Eng. Chem. Res. 2005, 44, 1557–1564. [Google Scholar] [CrossRef]
- Maring, B.J.; Webley, P.A. A new simplified pressure/vacuum swing adsorption model for rapid adsorbent screening for CO2 capture applications. Int. J. Greenh. Gas Control 2013, 15, 16–31. [Google Scholar] [CrossRef]
- Burns, T.D.; Pai, K.N.; Subraveti, S.G.; Collins, S.P.; Krykunov, M.; Rajendran, A.; Woo, T.K. Prediction of MOF Performance in Vacuum Swing Adsorption Systems for Postcombustion CO2 Capture Based on Integrated Molecular Simulations, Process Optimizations, and Machine Learning Models. Environ. Sci. Technol. 2020, 54, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Ben-Mansour, R.; Qasem, N.A.A. An efficient temperature swing adsorption (TSA) process for separating CO2 from CO2/N2 mixture using Mg-MOF-74. Energy Convers. Manag. 2018, 156, 10–24. [Google Scholar] [CrossRef]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101 (Cr)-PEI-800 and mmen-Mg-2 (dobpdc) MOF Adsorbents (vol 56, pg 750, 2017). Ind. Eng. Chem. Res. 2020, 59, 503–505. [Google Scholar] [CrossRef] [Green Version]
- Qasem, N.A.; Ben-Mansour, R. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Du, W.; Alkebsi, K.A.M. Model Predictive Control and Optimization of Vacuum Pressure Swing Adsorption for Carbon Dioxide Capture. In Proceedings of the 2017 6th International Symposium on Advanced Control of Industrial Processes (AdCONIP), Taipei, Taiwan, 28–31 May 2017; pp. 412–417. [Google Scholar]
- Durán, I.; Rubiera, F.; Pevida, C. Vacuum swing CO2 adsorption cycles in Waste-to-Energy plants. Chem. Eng. J. 2020, 382, 122841. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Schaef, H.T.; Kumar, A.; Lusi, M.; Pham, T.; Forrest, K.A.; Space, B.; Xu, W.; Halder, G.J. Hydrophobic pillared square grids for selective removal of CO2 from simulated flue gas. Chem. Commun. 2015, 51, 15530–15533. [Google Scholar] [CrossRef]
- Khraisheh, M.; Almomani, F.; Walker, G. Solid Sorbents as a Retrofit Technology for CO2 Removal from Natural Gas Under High Pressure and Temperature Conditions. Sci. Rep. 2020, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madden, D.G.; O’Nolan, D.; Chen, K.-J.; Hua, C.; Kumar, A.; Pham, T.; Forrest, K.A.; Space, B.; Perry, J.J.; Khraisheh, M. Highly selective CO2 removal for one-step liquefied natural gas processing by physisorbents. Chem. Commun. 2019, 55, 3219–3222. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, R.T.; Pai, K.N.; Subraveti, S.G.; Rajendran, A. Improving the performance of vacuum swing adsorption based CO2 capture under reduced recovery requirements. Int. J. Greenh. Gas Control 2020, 93, 102902. [Google Scholar] [CrossRef]
- Plaza, M.; Ferreira, A.; Santos, J.; Ribeiro, A.; Müller, U.; Trukhan, N.; Loureiro, J.; Rodrigues, A. Propane/propylene separation by adsorption using shaped copper trimesate MOF. Microporous Mesoporous Mater. 2012, 157, 101–111. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Belmabkhout, Y.; Cadiau, A.; Adil, K.; Shekhah, O.; Shkurenko, A.; Barbour, L.J.; Eddaoudi, M. A fine-tuned fluorinated MOF addresses the needs for trace CO2 removal and air capture using physisorption. J. Am. Chem. Soc. 2016, 138, 9301–9307. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.J.; Daniels, E.A.; Curtin, T.; Perry IV, J.J.; Zaworotko, M.J. Direct air capture of CO2 by physisorbent materials. Angew. Chemie Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Al-Sehemi, A.G.; Assiri, M.A.; Abdul Kareem, F.A.; Mukhtar, A.; Ayoub, M.; Gonfa, G. Influence of post-synthetic graphene oxide (GO) functionalization on the selective CO2/CH4 adsorption behavior of MOF-200 at different temperatures; an experimental and adsorption isotherms study. Microporous Mesoporous Mater. 2020, 296, 110002. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Assiri, M.A.; Al-Sehemi, A.G.; Gonfa, G.; Mukhtar, A.; Abdul Kareem, F.A.; Ayoub, M.; Saqib, S.; Mellon, N.B. Synthesis and characterization of mesoporous MOF UMCM-1 for CO2/CH4 adsorption; an experimental, isotherm modeling and thermodynamic study. Microporous Mesoporous Mater. 2020, 294, 109844. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Assiri, M.A.; Al-Sehemi, A.G.; Sagir, M.; Abdul Kareem, F.A.; Elkhalifah, A.E.I.; Mukhtar, A.; Gonfa, G. Synthesis, and characterization of metal-organic frameworks -177 for static and dynamic adsorption behavior of CO2 and CH4. Microporous Mesoporous Mater. 2019, 288, 109569. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Wang, X.; Zhou, W.; Jia, L.; Li, J.; Chen, B. Kinetic separation of propylene over propane in a microporous metal-organic framework. Chem. Eng. J. 2018, 354, 977–982. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]










| Cycle Step | Cycle Time (s) | Pressure (kPa) | Flow Rate (SLPM) | Composition | |
|---|---|---|---|---|---|
| PROPYNE | PROPYLENE | ||||
| Adsorption | 800 | 250 | 1.2 | 0.7 | 0.30 |
| Depressurization | 100 | 100 | - | ||
| Rinse | 600 | 100 | 1.2 | 1.0 | - |
| Countercurrent Blowdown | 100 (150–250) * | 250 down to 10 | - | - | - |
| Low pressure Purge | 600 | 10 | 1 | 1.0 | |
| Countercurrent Pressurization | 50 | 10–250 | 1.2 | - | 1.0 |
| Material | Adsorption Uptake (mmol·g−1) | Ref | Temp (K) | Pressure (kPa) |
|---|---|---|---|---|
| propyne | ||||
| SIFSIX-3-Ni | 3.32 | This study | 300 | 10 |
| SIF-Six-2-Cu-i | 1.73 | [8] | 273 | 0.1 |
| SIFSIX-3-Ni | 2.7 | [8] | 273 | 0.1 |
| SIFSIX-1-Cu | 0.19 | [12] | 298 | 0.1 |
| SIFSIX-2-Cu-i | 0.2 | [12] | 298 | 0.1 |
| SIFSIX-3-Ni | 2.65 | [12] | 298 | 0.1 |
| [Cu(dhbc)2(4,4′-bipy)] | 0.25 | [9] | 298 | 0.1 |
| NK-MOF-Ni | 1.83 | [8] | 273 | 0.1 |
| NK-MOF-Cu | 1.76 | [8] | 273 | 0.1 |
| Equation | Parameter Definition | Equation | |
|---|---|---|---|
| Langmuir isotherm | Q (mmol·g−1): adsorption capacity; qsat (mmol·g−1): equilibrium uptake capacity of the gas species; P: system pressure (kPa); kl: isotherm constant related to the energy of adsorption. | (1) | |
| Separation Factor | Rl separation factor | (2) | |
| Toth Isotherm | kt and n are Toth constants specific for adsorbate-adsorbent pairs; n indicates the affinity of the adsorption | (3) | |
| average absolute relative deviation | Qpred: predicted amounts; Qexp values of Q obtained experimentally; N: number of the experimental data points used in the isotherm fit. | (4) | |
| Isosteric heats of sorption | P: pressure; T: temperatures; qsa: saturated equilibrium uptake amount (mmol·g−1). | (5) | |
| Purity | Fi: the molar flow rate of component i (C3H6 and C3H4) (mol s−1); QF: the volumetric flow rate at the bed outlet (m3 s−1); | (6) | |
| Recovery | t: end of purge cycle; QF: the volumetric flow rate at the bed outlet (m3 s−1) | (7) | |
| Productivity | MCO2: the molecular weight of C3H6 (kg mol−1); tcycle: the total time of one repeated cycle (h); [purge, blowdown, rinse]; ms: the adsorbent mass (kg) | (8) | |
| Power consumption | η: the compressor efficiency; γ: the specific heat capacity of the gas, C: the gas concentration (mol m−3); QF: the volumetric flow rate at the bed outlet (m3 s−1); εtot: the total bed porosity | (9) |
| Langmuir | Toth | ||||
|---|---|---|---|---|---|
| Parameter | C3H4 | C3H6 | Parameter | C3H4 | C3H6 |
| qsat (mmol/g) | 3.32 | 2.93 | qsat (mmol/g) | 4.09 | 3.98 |
| kl | 0.23 | 0.21 | kt | 0.042 | 0.076 |
| Rl | 0.82 | 0.65 | n | 0.203 | 0.019 |
| AARD (%) | 13.7 | 12.2 | AARD (%) | 0.03 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khraisheh, M.; AlMomani, F.; Walker, G. High Purity/Recovery Separation of Propylene from Propyne Using Anion Pillared Metal-Organic Framework: Application of Vacuum Swing Adsorption (VSA). Energies 2021, 14, 609. https://doi.org/10.3390/en14030609
Khraisheh M, AlMomani F, Walker G. High Purity/Recovery Separation of Propylene from Propyne Using Anion Pillared Metal-Organic Framework: Application of Vacuum Swing Adsorption (VSA). Energies. 2021; 14(3):609. https://doi.org/10.3390/en14030609
Chicago/Turabian StyleKhraisheh, Majeda, Fares AlMomani, and Gavin Walker. 2021. "High Purity/Recovery Separation of Propylene from Propyne Using Anion Pillared Metal-Organic Framework: Application of Vacuum Swing Adsorption (VSA)" Energies 14, no. 3: 609. https://doi.org/10.3390/en14030609
APA StyleKhraisheh, M., AlMomani, F., & Walker, G. (2021). High Purity/Recovery Separation of Propylene from Propyne Using Anion Pillared Metal-Organic Framework: Application of Vacuum Swing Adsorption (VSA). Energies, 14(3), 609. https://doi.org/10.3390/en14030609