Impact of Attrition Ball-Mill on Characteristics and Biochemical Methane Potential of Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Food Waste and Seed Sludge
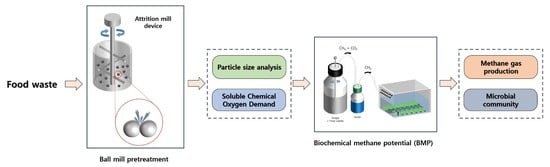
2.2. Attrition Mill Pretreatment
2.3. Biochemical Methane Potential (BMP) Test
2.4. Analytical Methods
3. Results and Discussion
3.1. Effects of Ball-Mill Pretreatment on Food Waste Properties
3.2. Effect of Ball-Mill Pretreatment on the BMP of Food Waste
3.3. Microbial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| TS | Total solid |
| WWTP | Waste water treatment plant |
| TCOD | Total chemical oxygen demand |
| SCOD | Soluble chemical oxygen demand |
| VS | Volatile solid |
| VFAs | Volatile fatty acids |
| MPS | Mean particle size |
| BMP | Biochemical methane potential |
| TVFAs | Total volatile fatty acids |
References
- Browne, J.; Murphy, J. Assessment of the resource associated with biomethane from food waste. Appl. Energy 2013, 104, 170–177. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2016; Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; p. 200. [Google Scholar]
- WRAP. Strategies to Achieve Economic and Environmental Gains by Reducing Food Waste; WRAP: Banbury, UK, 2015. [Google Scholar]
- Dev, S.; Saha, S.; Kurade, M.B.; Salama, E.; El-Dalatony, M.M.; Ha, G.; Chang, S.W.; Jeon, B. Perspective on anaerobic digestion for biometahanation in cold environments. Renew. Sustain. Energy Rev. 2019, 103, 85–95. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Increasing Profits in Food Waste Biorefinery—A Techno-Economic Analysis. Energies 2018, 11, 1551. [Google Scholar] [CrossRef] [Green Version]
- Ingrao, C.; Bacenetti, J.; Adamczyk, J.; Ferrante, V.; Messineo, A.; Huisingh, D. Investigating energy and environmental issues of agro-biogas derived energy systems; A comprehensive review of Life Cycle Assessments. Renew. Energy 2019, 136, 296–307. [Google Scholar] [CrossRef]
- Amaral, A.F.; Previtali, D.; Bassani, A.; Italiano, C.; Palella, A.; Pino, L.; Vita, A.; Bozzano, G.; Pirola, C.; Manenti, F. Biogas beyond CHP: The HPC (heat, power & chemicals) process. Energy 2020, 203, 117820. [Google Scholar]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Christopoulou, N.; Goumenaki, M. Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl. Energy 2007, 84, 646–663. [Google Scholar] [CrossRef]
- Igoni, A.H.; Ayotamuno, M.; Eze, C.; Ogaji, S.; Probert, S. Designs of anaerobic digesters for producing biogas from municipal solid-waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Perkoulidis, G. A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour. Technol. 2009, 100, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas Production from Anaerobic Co-Digestion of Spent Mushroom Substrate with Different Livestock Manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014, 152, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Merrylin, J.; Kumar, S.A.; Kaliappan, S.; Yeom, I.; Banu, J.R. Biological pretreatment of non-flocculated sludge augments the biogas production in the anaerobic digestion of the pretreated waste activated sludge. Environ. Technol. 2013, 34, 2113–2123. [Google Scholar] [CrossRef]
- Yunqin, L.; Dehan, W.; Lishang, W. Biological pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. Waste Manag. Res. 2010, 28, 800–810. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Lee, J.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy 2017, 137, 479–486. [Google Scholar] [CrossRef]
- Cai, M.; Liu, J.; Wei, Y. Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Potassium Hydroxyde Pre-Treatment Enhances Methane Yield from Giant Reed (Arundo donax L.). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Appels, L.; Van Assche, A.; Willems, K.; Degrève, J.; Van Impe, J.; Dewil, R. Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 4124–4130. [Google Scholar] [CrossRef]
- Naran, E.; Toor, U.A.; Kim, D.J. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016, 113, 17–21. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Cheng, J.; Hua, J.; Dong, H.; Zhou, J.; Li, Y.-Y. Improving fermentative methane production of glycerol trioleate and food waste pretreated with ozone through two-stage dark hydrogen fermentation and anaerobic digestion. Energy Convers. Manag. 2020, 203, 112225. [Google Scholar] [CrossRef]
- Shapiro, H.; Siegel, J.B. How ‘the crowd’ is tackling a silent killer. Nature 2018, 562, 7727. [Google Scholar]
- Achinas, S.; Euverink, G.J.W. Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material. Energies 2019, 12, 217. [Google Scholar] [CrossRef] [Green Version]
- Carballa, M.; Manterola, G.; Larrea, L.; Ternes, T.; Omil, F.; Lema, J.M. Influence of ozone pre-treatment on sludge anaerobic digestion: Removal of pharmaceutical and personal care products. Chemosphere 2007, 67, 1444–1452. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Nakhla, G.; Ray, M.B. Thermo-oxidative pretreatment of municipal waste activated sludge for volatile sulfur compounds removal and enhanced anaerobic digestion. Chem. Eng. J. 2011, 174, 166–174. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-K.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Gu, Y.M.; Byun, H.R.; Kim, Y.-H.; Park, D.-Y.; Lee, J.H. Assessing the potential of facile biofuel production from corn stover using attrition mill treatment. Water Energy Nexus 2019, 2, 46–49. [Google Scholar] [CrossRef]
- Kwon, B.G.; Na, S.H.; Lim, H.J.; Lim, C.S. Slurry phase decomposition of food waste by using various microorganisms. J. Korean Soc. Environ. Eng. 2014, 36, 303–310. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Kim, T.H.; Choi, W.I. Impact of planetary ball mills on corn stover characteristics and enzymatic digestibility depending on grinding ball properties. Bioresour. Technol. 2017, 241, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- 5220 B Open Reflux Method. In Standard Methods for the Examinations of Water and Waterwaters; American Pubilic Health Association: Washington, DC, USA, 2005.
- Lettinga, G.; Rebac, S.; Parshina, S.; Nozhevnikova, A.; van Lier, J.; Stams, A.J.M. High-rate anaerobic treatment of wastewater at low temperatures. Appl. Environ. Microbiol. 1999, 65, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Choi, O.; Pandey, A.; Kim, Y.G.; Joo, J.S.; Sang, B.-I. Simultaneous production of methane and acetate by thermophilic mixed culture from carbon dioxide in bioelectrochemical system. Bioresour. Technol. 2019, 281, 474–479. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Jeong, S.; Chandran, K.; Park, K.Y. Interactions between substrate characteristics and microbial communities on biogas production yield and rate. Bioresour. Technol. 2020, 303, 122934. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Castenholz, R.W.; Garrity, G.M. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Dürre, P. Handbook on Clostridia; Taylor & Francis: Boca Raton, FL, USA, 2005; p. 903. [Google Scholar]
- Kim, B.H.; Gadd, G.M. Bacterial Physiology and Metabolism; Cambridge University Press: Cambridge, NY, USA, 2008; Volume xxii, p. 529. [Google Scholar]
- Kotsyurbenko, O.R.; Chin, K.J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Eyice, O.; Cetecioglu, Z.; Plaza, E. The study of structure of anaerobic granules and methane producing pathways of pilot-scale UASB reactors treating municipal wastewater under sub-mesophilic conditions. Bioresour. Technol. 2019, 290, 121733. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. The Prokaryotes: Deltaproteobacteria and Epsilonproteobacteria, 4th ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Rosenberg, E. The Prokaryotes: Gammaproteobacteria, 4th ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Parawira, W.; Read, J.S.; Mattiasson, B.; Björnsson, L. Energy production from agricultural residues: High methane yields in pilot-scale two-stage anaerobic digestion. Biomass Bioenergy 2008, 32, 44–50. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Y.; Zhao, P.; Li, Q.X.; Guo, S.; Chen, C. Potential and optimization of two-phase anaerobic digestion of oil refinery waste activated sludge and microbial community study. Sci. Rep. 2016, 6, 38245. [Google Scholar] [CrossRef] [Green Version]



| Classification | Percentage (% Wet Weight) | Content in Each Category (% Wet Weight) |
|---|---|---|
| Vegetables | 50 | Chinese cabbage (50), cabbage (25), lettuce (25) |
| Fruits | 18 | Apple (25), mandarin (25), pear (25), persimmon (25) |
| Fish meat | 16 | Ham (40), fish cake (30), sausage (30) |
| Cereals | 16 | Rice (50), pasta (25), ramen (25) |
| Food Waste | Seed Sludge | |
|---|---|---|
| TCOD (g/L) 1 | 30 | 28.4 |
| SCOD (g/L) 2 | 19.1 | 0.727 |
| TS (g/L) 3 | 30 | 16 |
| VS (g/L) 4 | 28.1 | 12 |
| VS/TS (%) | 93.8 | 71.5 |
| pH | - | 8.23 |
| Speed (rpm) | Time (min) | MPS 1 (µm) | SCOD (g O2/L) | |
|---|---|---|---|---|
| Untreated | 0 | 0 | 834 ± 162 | 19.1 ± 0.1 |
| M1 | 150 | 10 | 550 ±177 | 19.8 ± 0.3 |
| M2 | 225 | 10 | 479 ±149 | 20.5 ± 0.1 |
| M3 | 300 | 10 | 241 ± 16.9 | 22.4 ± 0.0 |
| M4 | 300 | 20 | 212 ± 9.83 | 22.2 ± 0.2 |
| M5 | 300 | 30 | 176 ± 15.6 | 24.6 ± 0.2 |
| 0 Day | 1 Day | 4 Day | 7 Day | |
|---|---|---|---|---|
| Untreated | 100 | 1130 | 655 | 145 |
| M1 | 185 | 1596 | 518 | 22 |
| M2 | 101 | 1798 | 594 | 391 |
| M3 | 103 | 1832 | 629 | 460 |
| M4 | 98 | 1867 | 613 | 489 |
| M5 | 113 | 1921 | 639 | 592 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.M.; Park, S.Y.; Park, J.Y.; Sang, B.-I.; Jeon, B.S.; Kim, H.; Lee, J.H. Impact of Attrition Ball-Mill on Characteristics and Biochemical Methane Potential of Food Waste. Energies 2021, 14, 2085. https://doi.org/10.3390/en14082085
Gu YM, Park SY, Park JY, Sang B-I, Jeon BS, Kim H, Lee JH. Impact of Attrition Ball-Mill on Characteristics and Biochemical Methane Potential of Food Waste. Energies. 2021; 14(8):2085. https://doi.org/10.3390/en14082085
Chicago/Turabian StyleGu, Yang Mo, Seon Young Park, Ji Yeon Park, Byoung-In Sang, Byoung Seong Jeon, Hyunook Kim, and Jin Hyung Lee. 2021. "Impact of Attrition Ball-Mill on Characteristics and Biochemical Methane Potential of Food Waste" Energies 14, no. 8: 2085. https://doi.org/10.3390/en14082085
APA StyleGu, Y. M., Park, S. Y., Park, J. Y., Sang, B. -I., Jeon, B. S., Kim, H., & Lee, J. H. (2021). Impact of Attrition Ball-Mill on Characteristics and Biochemical Methane Potential of Food Waste. Energies, 14(8), 2085. https://doi.org/10.3390/en14082085