Analytics for Recovery and Reuse of Solid Wastes from Refineries
Abstract
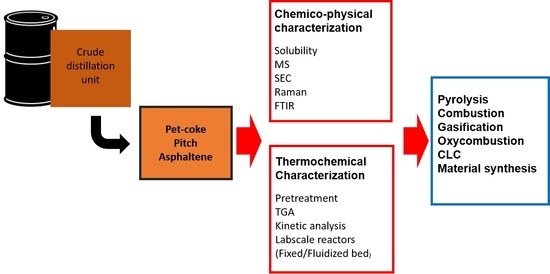
:1. Introduction
2. New Perspectives for Low Valuable Products of the Oil Refinery Industry
3. Methods for Chemico-Physical Characterization
3.1. Terminology for Refinery Residues
3.2. Solubility as a Characterization Technique
3.3. Mass Spectrometry (MS)
3.4. Size Exclusion Chromatography (SEC)
3.5. Raman Spectroscopy
3.6. FTIR Spectroscopy
4. Experimental Method for Investigating Thermochemical Conversion
4.1. Pretreatment and Physico-Chemical Characterization of the Solid
- The fuel, char and the ash samples are analysed by proximate and ultimate analysis, Scanning Electron Microscope- Energy Dispersive X-Ray (SEM-EDX), Inductively coupled plasma (ICP), X-ray diffraction (XRD), in some cases also porosimetric analysis.
- In case of metal rich samples, it is interesting to understand the stability of these metals throughout thermochemical processes. It is useful to calculate partitioning factors according to the formula: , where and . are the concentration of the metal in the examined sample and in the raw material, while and . are the concentration of a reference tracer metal. In particular, Ni can be used as a tracer thanks to its good thermal stability. Partitioning factors below unity indicate a release of the given metals with volatile products, while partitioning factors close to unity indicate that the metal is retained in the solid.
4.2. Thermogravimetric Analysis (TGA)
- TG curves, reporting the mass loss as a function of time/temperature,
- derivative thermogravimetry (DTG) curves, reporting derivative of the mass loss with respect to time,
- DSC profiles,
- Profiles of the main gaseous products evolved (by MS or FTIR).
- the carbon conversion degree f = (mo − m)/(mo − m∞) where mo and m∞ are the sample’s mass at the beginning and at the end of the test (the residual ash) and m is the mass at any given time.
- the instantaneous rate of carbon conversion, df/dt, versus f,
- the reaction rate averaged over a given interval of conversion (often 50%) Rav,
- Arrhenius plots of ln(Rav) as a function of 1/T for a fixed value of reactant concentration,
- Plots of ln(Rav) as a function of ln(pg), where pg is the value of the partial pressure of the reactant (O2, CO2, or H2O).
4.3. Pyrolysis in Lab Scale Reactors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirbas, A.; Acar, S.; Horasan, B.Y.; Alalayah, W.M. Analysis of petcoke from low grade oily sludge of refinery. Pet. Sci. Technol. 2018, 36, 904–909. [Google Scholar] [CrossRef]
- Yankovsky, S.A.; Kuznetsov, G.V.; Tolokolnikov, A.A.; Cherednik, I.V.; Ivanov, A.A. Experimental study of the processes of reducing the formation of sulfur oxides during the co-combustion of particles of metalignitous coal and wood processing waste. Fuel 2021, 291, 120233. [Google Scholar] [CrossRef]
- Yankovsky, S.A.; Kuznetsov, G.V. Physicochemical Transformations of Mixed Fuels Based on Typical Coals and Wood upon Heating. Solid Fuel Chem. 2019, 53, 22–28. [Google Scholar] [CrossRef]
- Georgiopoulou, M.; Lyberatos, G. Life cycle assessment of the use of alternative fuels in cement kilns: A case study. J. Environ. Manag. 2018, 216, 224–234. [Google Scholar] [CrossRef]
- Oggioni, G.; Riccardi, R.; Toninelli, R. Eco-efficiency of the world cement industry: A data envelopment analysis. Energy Policy 2011, 39, 2842–2854. [Google Scholar] [CrossRef]
- Sarma, B.; Cramb, A.W.; Fruehan, R.J. Reduction of FeO in smelting slags by solid carbon: Experimental results. Metall. Mater. Trans. B 1996, 27, 717–730. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Theofilou, C. Green energy at cement kiln in cyprus-use of sewage sludge as a conventional fuel substitute. Renew. Sustain. Energy Rev. 2008, 12, 531–541. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=PET&s=MCKEXUS1&f=M (accessed on 2 April 2022).
- Ren, H.F.; Jin, B.S.; Zhong, Z.P.; Yang, Y.P.; Cai, S. Experimental research on combustion of petroleum coke mixed with coal powder. Ranshao Kexue Yu Jishu/J. Combust. Sci. Technol. 2004, 10, 554–558. [Google Scholar]
- Wang, J.; Anthony, E.J.; Abanades, J.C. Clean and efficient use of petroleum coke for combustion and power generation. Fuel 2004, 83, 1341–1348. [Google Scholar] [CrossRef]
- Mi, T.; Wu, Z.S.; Shen, B.X.; Chen, H.P.; Liu, D.C. An experimental study of combustion characteristics of petroleum coke. Dev. Chem. Eng. Miner. Processing 2002, 10, 601–614. [Google Scholar] [CrossRef]
- Shpirt, M.Y.; Goryunova, N.P.; Zekel, L.A.; Golovin, G.S.; Kovil, R.Z. Vanadium compounds in petroleum coke and coke gasification and combustion products. Khimiya Tverdogo Topliva 1998, 2, 87–92. [Google Scholar]
- Legros, R.; Lim, C.J.; Brereton, C.M.H.; Grace, J.R. Circulating fluidized bed combustion of pitches derived from heavy oil upgrading. Fuel 1991, 70, 1465–1471. [Google Scholar] [CrossRef]
- Murugan, P.; Mahinpey, N.; Mani, T. Thermal cracking and combustion kinetics of asphaltenes derived from Fosterton oil. Fuel Process. Technol. 2009, 90, 1286–1291. [Google Scholar] [CrossRef]
- Benbouzid, M.; Hafsia, S. Thermal and kinetic analyses of pure and oxidized bitumens. Fuel 2008, 87, 1585–1590. [Google Scholar] [CrossRef]
- Yang, X.; Gates, I.D. Combustion Kinetics of Athabasca Bitumen from 1D Combustion Tube Experiments. J. Nat. Resour. Res. 2009, 18, 193–211. [Google Scholar] [CrossRef]
- Fermoso, J.; Arias, B.; Plaza, M.G.; Pevida, C.; Rubiera, F.; Pis, J.J.; Garcia-Pena, F.; Casero, P. High-pressure co-gasification of coal with biomass and petcoke. Fuel Process. Technol. 2009, 90, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Moreno, T.; Querol, X.; Alastuey, A.; de la Rosa, J.; Sánchez de la Campa, A.M.; Minguillón, M.; Gibbons, W. Variations in vanadium, nickel and lanthanoid element concentrations in urban air. Sci. Total Environ. 2010, 408, 4569–4579. [Google Scholar] [CrossRef]
- Banik, S.; Pisupati, S.V. Effects of pressure and CO concentration on vanadium, nickel and iron phase transformations for petcoke slag viscosity correlation development. Fuel 2019, 253, 238–248. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Nie, J.; Yang, W.; Xu, L.; Sun, L. Migration and transformation of vanadium and nickel in high sulfur petcoke during gasification processes. Energies 2018, 11, 2158. [Google Scholar] [CrossRef] [Green Version]
- Jaygopal, J.; Palappan, K.G.; Lakshminarasimhan, M.; Rajavel, M.; Suresh, S. Experimental investigations of vanadium and nickel distribution while firing petcoke in a circulating fluidised bed test facility. Int. J. Oil Gas Coal Technol. 2019, 20, 81–96. [Google Scholar] [CrossRef]
- Senneca, O.; Chirone, R.; Cortese, L.; Salatino, P. Pyrolysis and combustion of a solid refinery waste. Fuel 2020, 267, 117258. [Google Scholar] [CrossRef]
- Urciuolo, M.; Solimene, R.; Ammendola, P.; Krusch, S.; Scherer, P.; Chirone, R.; Senneca, O. On the agglomeration tendency of carbonaceous fuels in fluidized beds. Fuel 2020, 277, 118187. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S.; Hills, C.D. Microbial removal of sulphur from petcoke (petcoke). Fuel 2019, 235, 1501–1505. [Google Scholar] [CrossRef]
- Agarwal, P.; Sharma, D.K. Studies on the Desulfurization of Petcoke by Organorefining and Other Chemical and Biochemical Techniques under Milder Ambient Pressure Conditions. Pet. Sci. Technol. 2011, 29, 1482–1493. [Google Scholar] [CrossRef]
- Al-Haj-Ibrahim, H.; Morsi, B.I. Desulfurization of Petcoke: A Review. Ind. Eng. Chem. Res. 1992, 31, 1835–1840. [Google Scholar] [CrossRef]
- Ba, Z.; Zhao, J.; Li, C.; Huang, J.; Fang, Y.; Zhang, L.; Wang, Q. Developing efficient gasification technology for high-sulfur petcoke to hydrogen-rich syngas production. Fuel 2020, 267, 117170. [Google Scholar] [CrossRef]
- Okeke, I.J.; Adams, T.A. Combining petcoke and natural gas for efficient liquid fuels production. Energy 2018, 163, 426–442. [Google Scholar] [CrossRef]
- Al-Zareer, M.; Dincer, I.; Rosen, M.A. Production of hydrogen-rich syngas from novel processes for gasification of petcokes and coals. Int. J. Hydrogen Energy 2020, 45, 1577–11592. [Google Scholar] [CrossRef]
- Salatino, P.; Senneca, O. Plant and Process for the Looping Combustion of Solid Carbon Containing. Fuels. Patent WO 2010/026259, 11 March 2010. [Google Scholar]
- Korus, A.; Klimanek, A.; Sładek, S.; Szlęk, A.; Tilland, A.; Bertholin, S.; Haugen, N.E.L. Kinetic parameters of petroleum coke gasification for modelling chemical-looping combustion systems. Energy 2021, 232, 120935. [Google Scholar] [CrossRef]
- Cerciello, F.; Coppola, A.; Lacovig, P.; Senneca, O.; Salatino, P. Characterization of surface-oxides on char under periodically changing oxidation/desorption conditions. Renew. Sustain. Energy Rev. 2021, 137, 110453. [Google Scholar] [CrossRef]
- Cerciello, F.; Senneca, O.; Coppola, A.; Lacovig, P.; Salatino, P. The influence of temperature on the nature and stability of surface-oxides formed by oxidation of char. Renew. Sustain. Energy Rev. 2021, 137, 110595. [Google Scholar] [CrossRef]
- Apicella, B.; Russo, C.; Di Blasi, A.; Mennella, V.; Senneca, O.; Cerciello, F.; Ciajolo, A. Nano-restructuration of carbon materials under high temperature heat treatment for environmental application and energy storage. Chem. Eng. Trans. 2019, 73, 91–96. [Google Scholar]
- Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Carbon Dots: A Unique Fluorescent Cocktail of Polycyclic Aromatic Hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef]
- Gargiulo, V.; Apicella, B.; Stanzione, F.; Tregrossi, A.; Millan, M.; Ciajolo, A.; Russo, C. Structural Characterization of Large Polycyclic Aromatic Hydrocarbons. Part 2: Solvent-Separated Fractions of Coal Tar Pitch and Naphthalene-Derived Pitch. Energy Fuels 2016, 30, 2574–2583. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Song, X.; Zhao, Z.; Zhang, N.; Hao, C. Pitch-derived carbon quantum dots as fluorescent probe for selective and sensitive detection of ferric ions and bioimaging. J. Photochem. Photobiol. A Chem. 2021, 412, 113253. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, M.; Fan, W.; Wang, X.; Tang, F.; Yang, C.; Dou, X.; Li, S.; Wang, Y.; Cao, Y. Facile One-Pot Conversion of Petroleum Asphaltene to High Quality Green Fluorescent Graphene Quantum Dots and Their Application in Cell Imaging. Part. Part. Syst. Charact. 2016, 33, 635–644. [Google Scholar] [CrossRef]
- Apicella, B.; Pré, P.; Rouzaud, J.N.; Abrahamson, J.; Vander Wal, R.L.; Ciajolo, A.; Tregrossi, A.; Russo, C. Laser-induced structural modifications of differently aged soot investigated by HRTEM. Combust. Flame 2019, 204, 13–22. [Google Scholar] [CrossRef]
- Wen, C.S.; Chilingarian, G.; Yen, T.F. Properties and structure of bitumens. In Bitumens, Asphalts and Tar Sands; Elsevier: Amsterdam, The Netherlands, 1978; Volume 7, pp. 155–190. [Google Scholar]
- Mullins, O.C.; Sheu, E.Y. (Eds.) Structures and Dynamics of Asphaltenes; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Mullins, O.C.; Sheu, E.Y.; Hammami, A.; Marshall, A.G. Asphaltenes, Heavy Oils, and Petroleomics; Springer Science and Business Media: New York, NY, USA, 2007. [Google Scholar]
- Yen, T.F.; Chilingarian, G.V. Asphaltenes and Asphalts, 2: Part B; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Speight, J.G. Petroleum Asphaltenes—Part 1: Asphaltenes, Resins and the Structure of Petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Mullins, O.C.; Martinez-Haya, B.; Marshall, A.G. Contrasting perspective on asphaltene molecular weight. Energy Fuels 2008, 22, 1765–1773. [Google Scholar] [CrossRef]
- Badre, S.; Carla Goncalves, C.; Norinaga, K.; Gustavson, G.; Mullins, O.C. Molecular size and weight of asphaltene and asphaltene solubility fractions fromcoals, crude oils and bitumen. Fuel 2006, 85, 1–11. [Google Scholar] [CrossRef]
- Kokal, S.L.; Selim, G.S. Asphaltenes: The Cholesterol of Petroleum. In Proceedings of the Middle East Oil Show, Manama, Bahrain, 11–14 March 1995. [Google Scholar] [CrossRef]
- Herod, A.A.; Bartle, K.D.; Kandiyoti, R. Comment on a paper by Mullins, Martinez-Haya and Marshall “Contrasting perspective on asphaltene molecular weight. This comment vs the overview of Herod, A.A., Bartle, K.D. and Kandiyoti, R”. Energy Fuels 2008, 22, 4312–4317. [Google Scholar] [CrossRef]
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Andrews, A.B.; Gross, L.; Pauchard, V.; Banerjee, S.; Mullins, O.C. Overview of asphaltene nanostructures and thermodynamic applications. Energy Fuels 2020, 34, 15082–15105. [Google Scholar] [CrossRef]
- Greinke, R.A. Kinetics of petroleum pitch polymerization by gel permeation chromatography. Carbon 1986, 24, 677–686. [Google Scholar] [CrossRef]
- Edwards, W.F.; Jin, L.; Thies, M.C. MALDI-TOF mass spectrometry: Obtaining reliable mass spectra for insoluble carbonaceous pitches. Carbon 2003, 41, 2761–2768. [Google Scholar] [CrossRef]
- Russo, C.; Ciajolo, A.; Stanzione, F.; Tregrossi, A.; Oliano, M.M.; Carpentieri, A.; Apicella, B. Investigation on chemical and structural properties of coal- and petroleum-derived pitches and implications on physico-chemical properties (solubility, softening and coking). Fuel 2019, 245, 478–487. [Google Scholar] [CrossRef]
- Marsh, H.; Cornford, C. Mesophase: The precursor to graphitizable carbon. In Petroleum Derived Carbons; American Chemical Society: Colombia, WA, USA, 1976; pp. 266–280. [Google Scholar]
- Edie, D.D. The effect of processing on the structure and properties of carbon fibers. Carbon 1998, 36, 345–362. [Google Scholar] [CrossRef]
- Beauharnois, M.E.; Edie, D.D.; Thies, M.C. Carbon fibers from mixtures of AR and supercritically extracted mesophases. Carbon 2001, 39, 2101–2111. [Google Scholar] [CrossRef]
- Wagner, M.H.; Jäger, H.; Letizia, I.; Wilhelmi, G. Quality assessment of binder pitches for carbon and graphite electrodes. Fuel 1988, 67, 792–797. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Bartle, K.D. Production of Mesophase Pitch from Coal Tar and Petroleum Pitches using Supercritical Fluid Extraction. Turk. J. Chem. 2002, 26, 417–424. [Google Scholar]
- Chwastiak, S.; Lewis, I.C. Solubility of Mesophase Pitch. Carbon 1978, 16, 156–157. [Google Scholar] [CrossRef]
- Gross, J.H. Mass Spectrometry: A Textbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cotter, R.J. Time-of-Flight Mass Spectrometry; ACS Professional Reference Books; American Chemical Society: Colombia, WA, USA, 1997. [Google Scholar]
- Awad, H.; Khamis, M.M.; El-Aneed, A. Mass spectrometry, review of the basics: Ionization. Appl. Spectrosc. Rev. 2015, 50, 158–175. [Google Scholar] [CrossRef]
- Vastola, F.J.; Mumma, R.O.; Pirone, A.J. Analysis of organic salts by laser ionization. Org. Mass Spectrom. 1979, 3, 101–104. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef]
- Apicella, B.; Carpentieri, A.; Alfè, M.; Barbella, R.; Tregrossi, A.; Pucci, P.; Ciajolo, A. Mass spectrometric analysis of large PAH in a fuel-rich ethylene flame. Proc. Combust. Inst. 2007, 31, 547–553. [Google Scholar] [CrossRef]
- Rizzi, A.; Cosmina, P.; Flego, C.; Montanari, L.; Seraglia, R.; Traldi, P. Laser desorption/ionization techniques in the characterization of high molecular weight oil fractions Part 1. Asphaltenes. J. Mass Spectrom. 2006, 41, 1232–1237. [Google Scholar] [CrossRef]
- Comisarow, M.B.; Marshall, A.G. Fourier Transform Ion Cyclotron Resonance Spectroscopy. Chem. Phys. Lett. 1974, 25, 282–283. [Google Scholar] [CrossRef]
- Robb, D.B.; Covey, T.R.; Bruins, A.P. Atmospheric pressure photoionization: An ionization method for liquid chromatography-mass spectrometry. Anal. Chem. 2000, 72, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.; Gutierrez, L.B.; Negrin, G.; Pereira, J.C.; Mendez, B.; Delolme, F.; Dessalces, G.; Broseta, D. Molecular weight of petroleum asphaltenes: A comparison between mass spectrometry and vapor pressure osmometry. Energy Fuels 2005, 19, 1548–1560. [Google Scholar] [CrossRef]
- Al-Muhareb, E.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. Characterization of petroleum asphaltenes by size exclusion chromatography, UV-fluorescence and mass spectrometry. Pet. Sci. Technol. 2007, 25, 81–91. [Google Scholar] [CrossRef]
- Hortal, A.R.; Martinez-Haya, B.; Lobato, M.D.; Pedrosa, J.M.; Lago, S. On the determination of molecular weight distributions of asphaltenes and their aggregates in laser desorption ionization experiments. J. Mass Spectrom. 2006, 41, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Sato, S.; Takanohashi, T.; Hunt, J.E.; Winans, R.E. Analysis of the molecular weight distribution of petroleum asphaltenes using laser desorption-mass spectrometry. Energy Fuels 2004, 18, 1405–1413. [Google Scholar] [CrossRef]
- Apicella, B.; Ciajolo, A.; Carpentieri, A.; Popa, C.; Russo, C. Characterization Techniques Coupled with Mathematical Tools for Deepening Asphaltene Structure. Fuels 2022, 3, 75–84. [Google Scholar] [CrossRef]
- Groenzin, H.; Mullins, O.C. Asphaltenes molecular size and structure. J. Phys. Chem. A 1999, 103, 11237. [Google Scholar] [CrossRef]
- Groenzin, H.; Mullins, O.C. Molecular size and structure of asphaltenes from various sources. Energy Fuels 2000, 14, 677–684. [Google Scholar] [CrossRef]
- Pomerantz, A.E.; Hammond, M.R.; Morrow, A.L.; Mullins, O.C.; Zare, R.N. Two-step laser mass spectrometry of asphaltenes. J. Am. Chem. Soc. 2008, 130, 7216. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.M.; Marshall, A.G.; Rodgers, R.P. Heavy Petroleum Composition. 4. Asphaltene Compositional Space. Energy Fuels 2013, 27, 1257–1267. [Google Scholar] [CrossRef]
- Apicella, B.; Alfè, M.; Ciajolo, A. Mass Spectrometric Advances in the Analysis of Large Aromatic Fractions of Heavy Fuel Oils and Carbon Particulates. Combust. Sci. Technol. 2010, 182, 640–652. [Google Scholar] [CrossRef]
- Apicella, B.; Alfè, M.; Amoresano, A.; Galano, E.; Ciajolo, A. Advantages and limitations of Laser desorption/ionization Mass Spectrometric techniques in the chemical characterization of complex carbonaceous materials. Int. J. Mass Spectrom. 2010, 295, 98–102. [Google Scholar] [CrossRef]
- Hortal, A.R.; Hurtado, P.; Martinez-Haya, B.M.; Mullins, O.C. Molecular-weight distributions of coal and petroleum asphaltenes from laser desorption/ionization experiments. Energy Fuels 2007, 21, 2863–2868. [Google Scholar] [CrossRef]
- Merdrignac, I.; Espinat, D. Physicochemical characterization of petroleum fractions: The state of the art. Oil Gas Sci. Technol.–Rev. IFP 2007, 62, 7–32. [Google Scholar] [CrossRef]
- Islas, C.A.; Suelves, I.; Millan, M.; Apicella, B.; Herod, A.A.; Kandiyoti, R. Matching average masses of pitch fractions of narrow polydispersity, derived from matrix-assisted laser desorption ionisation time-of-flight mass spectrometry, with the polystyrene calibration of SEC. J. Sep. Sci. 2003, 26, 1422–1428. [Google Scholar] [CrossRef]
- Karaka, F.; Islas, C.A.; Millan, M.; Behrouzi, M.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. The calibration of size exclusion chromatography columns: Molecular mass distributions of heavy hydrocarbon liquids. Energy Fuels 2004, 18, 778–788. [Google Scholar] [CrossRef]
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the Molecular Structures of Asphaltenes by Atomic Force Microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef]
- Cristadoro, A.; Kulkarni, S.U.; Burgess, W.A.; Cervo, E.G.; Räder, H.J.; Müllen, K.; Bruce, D.A.; Thies, M.C. Structural characterization of the oligomeric constituents of petroleum pitches. Carbon 2009, 47, 2358–2370. [Google Scholar] [CrossRef]
- Burgess, W.A.; Pittman, J.J.; Marcus, R.K.; Thies, M.C. Structural identification of the monomeric constituents of petroleum pitch. Energy Fuels 2010, 24, 4301–4311. [Google Scholar] [CrossRef]
- Zhang, W.; Andersson, J.T.; Rader, H.J.; Mullen, K. Molecular characterization of large polycyclic aromatic hydrocarbons in solid petroleum pitch and coal tar pitch by high resolution MALDI ToF MS and insights from ion mobility separation. Carbon 2015, 95, 672–680. [Google Scholar] [CrossRef]
- Sadao, M.; Barth, H.G. Size Exclusion Chromatography; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kandiyoti, R.; Herod, A.; Bartle, K.D.; Morgan, T.J. Solid Fuels and Heavy Hydrocarbon Liquids: Thermal Characterization and Analysis; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Herod, A.A.; Bartle, K.D.; Kandiyoti, R. Characterization of heavy hydrocarbons by chromatographic and mass spectrometric methods: An overview. Energy Fuels 2007, 21, 2176–2203. [Google Scholar] [CrossRef]
- Morgan, T.J.; Georg, A.; Alvarez, P.; Herod, A.A.; Millan, M.; Kandiyoti, R. Isolation of size exclusion chromatography elution-fractions of coal and petroleum-derived samples and analysis by laser desorption mass spectrometry. Energy Fuels 2009, 23, 6003–6014. [Google Scholar] [CrossRef]
- Karaca, F.; Morgan, T.J.; George, A.; Bull, I.D.; Herod, A.A.; Millan, M.; Kandiyoti, R. Molecular mass ranges of coal tar pitch fractions by mass spectrometry and size-exclusion chromatography. Rapid Commun. Mass Spectrom. 2009, 23, 2087–2098. [Google Scholar] [CrossRef]
- Gargiulo, V.; Apicella, B.; Alfè, M.; Russo, C.; Stanzione, F.; Tregrossi, A.; Amoresano, A.; Millan, M.; Ciajolo, A. Structural Characterization of Large Polycyclic Aromatic Hydrocarbons. Part 1: The Case of Coal Tar Pitch and Naphthalene-Derived Pitch. Energy Fuels 2015, 29, 5714–5722. [Google Scholar] [CrossRef]
- Apicella, B.; Barbella, R.; Ciajolo, A.; Tregrossi, A. Comparative analysis of the structure of carbon materials relevant in combustion. Chemosphere 2003, 51, 1063–1069. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Bouhadda, Y.; Bormann, D.; Sheu, E.; Bendedouch, D.; Krallafa, A.; Daaou, M. Characterization of Algerian Hassi-Messaoud asphaltene structure using Raman spectrometry and X-ray diffraction. Fuel 2007, 86, 1855–1864. [Google Scholar] [CrossRef]
- Dumont, M.; Chollon, G.; Dourges, M.A.; Pailler, R.; Bourrat, X.; Naslain, R.; Bruneel, J.L.; Couzi, M. Chemical, microstructural and thermal analyses of a naphthalene-derived mesophase pitch. Carbon 2002, 40, 1475–1486. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Origin of the 1150−cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 2001, 63, 121405. [Google Scholar] [CrossRef]
- Mallet-Ladeira, P.; Puech, P.; Weisbecker, P.; Vignoles, G.L.; Monthioux, M. Behavior of Raman D band for pyrocarbons with crystallite size in the 2–5 nm range. Appl. Phys. A 2014, 114, 759–763. [Google Scholar] [CrossRef]
- Puech, P.; Plewa, J.M.; Mallet-Ladeira, P.; Monthioux, M. Spatial confinement model applied to phonons in disordered graphene-based carbons. Carbon 2016, 105, 275–281. [Google Scholar] [CrossRef]
- Casiraghi, C.; Piazza, F.; Ferrari, A.C.; Grambole, D.; Robertson, J. Bonding in hydrogenated diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2005, 14, 1098–1102. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Gago, R.; Jiḿnez, I.; Camero, M.; Agulló-Rueda, F.; Gómez-Aleixandre, C. Hydrogen quantification in hydrogenated amorphous carbon films by infrared, Raman, and X-ray absorption near edge spectroscopies. J. Appl. Phys. 2009, 105, 093510. [Google Scholar] [CrossRef] [Green Version]
- Riedeman, J.S.; Kadasala, N.R.; Wei, A.; Kenttämaa, H.I. Characterization of Asphaltene Deposits by Using Mass Spectrometry and Raman Spectroscopy. Energy Fuels 2016, 30, 805–809. [Google Scholar] [CrossRef]
- Öner, F.O.; Yürüm, A.; Yürüma, Y. Structural characterization of semicokes produced from the pyrolysis of petroleum pitches. J. Anal. Appl. Pyrolysis 2015, 111, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, H.; Ibrahim, U.K.; Xue, W.; Liu, H.; Guo, A. The quantitative assessment of coke morphology based on the Raman spectroscopic characterization of serial petcokes. Fuel 2019, 246, 60–68. [Google Scholar] [CrossRef]
- Kostecki, R.; Tran, T.; Song, X.; Kinoshita, K.; McLarnon, F. Raman Spectroscopy and Electron Microscopy of Heat-Treated Petcokes for Lithium-Intercalation Electrodes. J. Electrochem. Soc. 1997, 144, 3111. [Google Scholar] [CrossRef]
- Dischler, B.; Bubenzer, A.; Koidl, P. Bonding in hydrogenated hard carbon studied by optical spectroscopy. Solid State Comun. 1983, 48, 105–108. [Google Scholar] [CrossRef]
- Centrone, A.; Brambilla, L.; Renouard, T.; Gherghel, L.; Mathis, C.; Mullen, K.; Zerbi, G. Structure of new carbonaceous material. The role of vibrational spectroscopy. Carbon 2005, 43, 1593–1609. [Google Scholar] [CrossRef]
- Calemma, V.; Iwanski, P.; Nali, M.; Scotti, R.; Montanari, L. Structural Characterization of Asphaltenes of Different Origins. Energy Fuels 1995, 9, 225–230. [Google Scholar] [CrossRef]
- Wilt, B.K.; Welch, W.T. Determination of Asphaltenes in Petroleum Crude Oils by Fourier Transform Infrared Spectroscopy. Energy Fuels 1998, 12, 1008–1012. [Google Scholar] [CrossRef]
- Aske, N.; Kallevik, H.; Sjoblom, J. Determination of Saturate, Aromatic, Resin, and Asphaltenic (SARA) Components in Crude Oils by Means of Infrared and Near-Infrared Spectroscopy. Energy Fuels 2001, 15, 1304–1312. [Google Scholar] [CrossRef]
- Guillén, M.D.; Iglesias, M.J.; Domínguez, A.; Blanco, C.G. Fourier transform infrared study of coal tar pitches. Fuel 1995, 74, 1595–1598. [Google Scholar] [CrossRef]
- Guillén, M.D.; Iglesias, M.J.; Domínguez, A.; Blanco, C.G. Semiquantitative FTIR Analysis of a Coal Tar Pitch and Its Extracts and Residues in Several Organic Solvents. Energy Fuels 1992, 6, 518–525. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Cazorla-Amorós, D.; Linares-Solano, A. Characterisation of coal tar pitches by thermal analysis, infrared spectroscopy and solvent fractionation. Fuel 2001, 80, 41–48. [Google Scholar] [CrossRef]
- Tzeng, S.S.; Pan, J.H. Oxidative stabilization of petroleum pitch at high pressure and its effects on the microstructure and carbon yield after carbonization/graphitization. Mater. Chem. Phys. 2002, 74, 214–221. [Google Scholar] [CrossRef]
- Menendez, J.A.; Pis, J.J.; Alvarez, R.; Barriocanal, C.; Canga, C.S.; Diez, M.A. Characterization of Petcoke as an Additive in Metallurgical Cokemaking. Influence on Metallurgical Coke Quality. Energy Fuels 1997, 11, 379–384. [Google Scholar] [CrossRef]
- Ristein, J.; Stief, R.T.; Ley, L.; Beyer, W. A comparative analysis of a-C:H by infrared spectroscopy and mass selected thermal effusion. J. Appl. Phys. 1998, 84, 3836–3847. [Google Scholar] [CrossRef]
- Jacob, W.; Unger, M. Experimental determination of the absorption strength of C–H vibrations for infrared analysis of hydrogenated carbon films. Appl. Phys. Lett. 1996, 68, 475–477. [Google Scholar] [CrossRef]
- Russo, C.; Stanzione, F.; Tregrossi, A.; Ciajolo, A. Infrared spectroscopy of some carbon based materials relevant in combustion: Qualitative and quantitative analysis of hydrogen. Carbon 2014, 74, 127–138. [Google Scholar] [CrossRef]
- Russo, C.; Tregrossi, A.; Ciajolo, A. Dehydrogenation and growth of soot in premixed flames. Proc. Comb. Inst. 2015, 35, 1803–1809. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Gartner, J.K.; Garcia-Perez, M.; Van Wie, B.J. Investigation of biomass char gasification kinetic parameters using a novel miniaturized educational system. BioResources 2019, 14, 3594–3614. [Google Scholar]
- Everson, R.C.; Neomagus, H.W.J.P.; Kaitano, R. The random pore model with intraparticle diffusion for the description of combustion of char particles derived from mineral- and inertinite rich coal. Fuel 2011, 90, 2347–2352. [Google Scholar] [CrossRef]
- Trommer, D.; Noembrini, F.; Fasciana, M.; Rodriguez, D.; Morales, A.; Romero, M.; Steinfeld, A. Hydrogen production by steam-gasification of petroleum coke using concentrated solar power—I. Thermodynamic and kinetic analyses. Int. J. Hydrogen Energy 2005, 30, 605–618. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Yao, Y.; Zhang, Y.; Cao, J. Steam gasification of coal char catalyzed by K2CO3 for enhanced production of hydrogen without formation of methane. Fuel 2009, 88, 1572–1579. [Google Scholar] [CrossRef]
- Afrooz, I.E.; Ching, D.L.C. A modified model for kinetic analysis of petroleum coke. Int. J. Chem. Eng. 2019, 2019, 2034983. [Google Scholar]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apicella, B.; Russo, C.; Senneca, O. Analytics for Recovery and Reuse of Solid Wastes from Refineries. Energies 2022, 15, 4026. https://doi.org/10.3390/en15114026
Apicella B, Russo C, Senneca O. Analytics for Recovery and Reuse of Solid Wastes from Refineries. Energies. 2022; 15(11):4026. https://doi.org/10.3390/en15114026
Chicago/Turabian StyleApicella, Barbara, Carmela Russo, and Osvalda Senneca. 2022. "Analytics for Recovery and Reuse of Solid Wastes from Refineries" Energies 15, no. 11: 4026. https://doi.org/10.3390/en15114026
APA StyleApicella, B., Russo, C., & Senneca, O. (2022). Analytics for Recovery and Reuse of Solid Wastes from Refineries. Energies, 15(11), 4026. https://doi.org/10.3390/en15114026