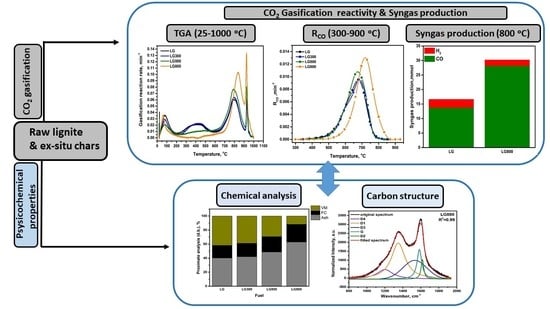
CO2 Gasification Reactivity and Syngas Production of Greek Lignite Coal and Ex-Situ Produced Chars under Non-Isothermal and Isothermal Conditions: Structure-Performance Relationships
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Analysis
2.2. Structural Characterization
2.3. Thermogravimetric Analysis
2.4. Gasification Experiments
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. Chemical Analysis
3.1.2. XRD Analysis
3.1.3. Raman Analysis
3.2. Gasification Reactivity
3.3. Syngas Production during Non-Isothermal Gasification Tests
Effect of CO2 Concentration on the Gasification Performance
3.4. Syngas Production during Isothermal CO2 Gasification Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in european countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef] [Green Version]
- Looney, B. Statistical Review of World Energy, 2020, 69th ed.; Bp: London, UK, 2020; Volume 69, p. 66. [Google Scholar]
- IEA. Coal 2020; IEA: Paris, France, 2020; p. 124. [Google Scholar]
- International Energy Agency. Energy Policies IEA Countries: Greece 2017 Review; IEA: Paris, France, 2017. [Google Scholar]
- Directorate-General for Research and Innovation (European Commission). Europe’s 2030 Climate and Energy Targets. Research & Innovation Actions; Directorate-General for Research and Innovation (European Commission): Bruxelles, Belgium, 2021; Volume 3. [Google Scholar]
- Pavloudakis, F.; Roumpos, C.; Karlopoulos, E.; Koukouzas, N. Sustainable rehabilitation of surface coal mining areas: The case of greek lignite mines. Energies 2020, 13, 3995. [Google Scholar] [CrossRef]
- Karasmanaki, E.; Ioannou, K.; Katsaounis, K.; Tsantopoulos, G. The attitude of the local community towards investments in lignite before transitioning to the post-lignite era: The case of Western Macedonia, Greece. Resour. Policy 2020, 68, 101781. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhao, R.; Wu, J. Gasification of shenhua bituminous coal with CO2: Effect of coal particle size on kinetic behavior and ash fusibility. Energies 2020, 13, 3313. [Google Scholar] [CrossRef]
- Kapusta, K. Effect of lignite properties on its suitability for the implementation of underground coal gasification (UCG) in selected deposits. Energies 2021, 14, 5816. [Google Scholar] [CrossRef]
- Zdeb, J.; Howaniec, N.; Smoliński, A. Utilization of carbon dioxide in coal gasification—An experimental study. Energies 2019, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Czajka, K.M. Gasification of coal by CO2: The impact of the heat transfer limitation on the progress, reaction rate and kinetics of the process. Energies 2021, 14, 5569. [Google Scholar] [CrossRef]
- Jewulski, J.; Skrzypkiewicz, M.; Struzik, M.; Lubarska-Radziejewska, I. Lignite as a fuel for direct carbon fuel cell system. Int. J. Hydrog. Energy 2014, 39, 21778–21785. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Mitsakis, P.; Mertzis, D.; Tsiakmakis, S.; Manara, P.; Samaras, Z. Bioenergy technology: Gasification with internal combustion engine application. Energy Procedia 2013, 42, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, N.; Fang, Y.; Fan, H.; Lei, Z.; Han, M. Journal of Materials Science & Technology Syngas production via coal char-CO 2 fluidized bed gasification and the effect on the performance of LSCFN//LSGM//LSCFN solid oxide fuel cell. J. Mater. Sci. Technol. 2018, 34, 403–408. [Google Scholar]
- Selvatico, D.; Lanzini, A.; Santarelli, M. Low Temperature Fischer-Tropsch fuels from syngas: Kinetic modeling and process simulation of different plant configurations. Fuel 2016, 186, 544–560. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Tsang, D.C.W.; Kwon, E.E.; Wang, C.H. Towards practical application of gasification: A critical review from syngas and biochar perspectives. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1165–1213. [Google Scholar] [CrossRef] [Green Version]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Renganathan, T.; Yadav, M.V.; Pushpavanam, S.; Voolapalli, R.K.; Cho, Y.S. CO2 utilization for gasification of carbonaceous feedstocks: A thermodynamic analysis. Chem. Eng. Sci. 2012, 83, 159–170. [Google Scholar] [CrossRef]
- Roncancio, R.; Gore, J.P. CO2 char gasification: A systematic review from 2014 to 2020. Energy Convers. Manag. X 2021, 10, 100060. [Google Scholar] [CrossRef]
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas production through gasification and cleanup for downstream applications—Recent developments. Fuel Process. Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Pohořelý, M.; Jeremiáš, M.; Svoboda, K.; Kameníková, P.; Skoblia, S.; Beňo, Z. CO2 as moderator for biomass gasification. Fuel 2014, 117, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, K.; Gökalp, I.; Jeyakumar, S. Estimation of synergetic effects of CO2 in high ash coal-char steam gasification. Appl. Therm. Eng. 2017, 110, 991–998. [Google Scholar] [CrossRef]
- Xiang, Y.; Cai, L.; Guan, Y.; Liu, W.; Cheng, Z.; Liu, Z. Study on the effect of gasification agents on the integrated system of biomass gasification combined cycle and oxy-fuel combustion. Energy 2020, 206, 118131. [Google Scholar] [CrossRef]
- Bielowicz, B. The suitability of Polish lignite for gasification. Clean Technol. Environ. Policy 2019, 21, 1115–1130. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhao, H.; Liu, X.; Li, Y.; Zhao, G.; Wang, Y.; Zeng, M. Effect of hydrothermal upgrading on the pyrolysis and gasification characteristics of baiyinhua lignite and a mechanistic analysis. Fuel 2020, 276, 1–12. [Google Scholar] [CrossRef]
- Skodras, G.; Nenes, G.; Zafeiriou, N. Low rank coal—CO2 gasification: Experimental study, analysis of the kinetic parameters by Weibull distribution and compensation effect. Appl. Therm. Eng. 2015, 74, 111–118. [Google Scholar] [CrossRef]
- Łabojko, G.; Kotyczka-Morańska, M.; Plis, A.; Ściazko, M. Kinetic study of polish hard coal and its char gasification using carbon dioxide. Thermochim. Acta 2012, 549, 158–165. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Fang, L. Evolution of char structure during non-isothermal low temperature pyrolysis of ZhunDong coal by microwave heating: A comparative study with conventional heating. J. Energy Inst. 2020, 93, 1195–1206. [Google Scholar] [CrossRef]
- Xi, J.; Liang, J.; Sheng, X.; Shi, L.; Li, S. Characteristics of lump lignite pyrolysis and the influence of temperature on lignite swelling in underground coal gasification. J. Anal. Appl. Pyrolysis 2016, 117, 228–235. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gokalp, I.; Bonifaci, E.; Merlo, N. Kinetics of steam and CO2 gasification of high ash coal-char produced under various heating rates. Fuel 2015, 154, 370–379. [Google Scholar] [CrossRef]
- Gomez, A.; Vargas, M.; Mahinpey, N. A theoretical model to estimate steam and CO2 gasification rates based on feedstock characterization properties. Fuel Process. Technol. 2016, 149, 187–194. [Google Scholar] [CrossRef]
- Zhu, S.; Bai, Y.; Hao, C.; Li, F.; Bao, W. Investigation into the structural features and gasification reactivity of coal chars formed in CO2 and N2 atmospheres. J. CO2 Util. 2017, 19, 9–15. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Schurz, M.; Steffen, K.; Meyer, B. Kinetic study on CO2 gasification of brown coal and biomass chars: Reaction order. Fuel 2016, 173, 311–319. [Google Scholar] [CrossRef]
- Edreis, E.M.A.; Luo, G.; Yao, H. Investigations of the structure and thermal kinetic analysis of sugarcane bagasse char during non-isothermal CO2 gasification. J. Anal. Appl. Pyrolysis 2014, 107, 107–115. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Wang, G.; Ning, X.; Wang, H.; Wang, P. Study on CO2 gasification reactivity of biomass char derived from high-temperature rapid pyrolysis. Appl. Therm. Eng. 2017, 121, 1022–1031. [Google Scholar] [CrossRef]
- Tong, W.; Liu, Q.; Yang, C.; Cai, Z.; Wu, H.; Ren, S. Effect of pore structure on CO2 gasification reactivity of biomass chars under high-temperature pyrolysis. J. Energy Inst. 2020, 93, 962–976. [Google Scholar] [CrossRef]
- Kirtania, K.; Bhattacharya, S. CO2 gasification kinetics of algal and woody char procured under different pyrolysis conditions and heating rates. ACS Sustain. Chem. Eng. 2015, 3, 365–373. [Google Scholar] [CrossRef]
- Parvez, A.M.; Afzal, M.T.; Victor Hebb, T.G.; Schmid, M. Utilization of CO2 in thermochemical conversion of biomass for enhanced product properties: A review. J. CO2 Util. 2020, 40, 101217. [Google Scholar] [CrossRef]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Chan, Y.H.; Syed Abdul Rahman, S.N.F.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Pollut. 2021, 278. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, A.; Binas, V.; Konsolakis, M. ScienceDirect Steam gasification of Greek lignite and its chars by co-feeding CO2 toward syngas production with an adjustable H2/CO ratio. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, W.; Cheng, Y.; Liu, Z.; Zhang, Q.; Zhao, K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel 2019, 239, 559–572. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, H.; Zeng, K.; Zhu, Y.; Hu, J.; Mao, Q.; Liu, Q.; Chen, H. Study on CO2 gasification of biochar in molten salts: Reactivity and structure evolution. Fuel 2019, 254, 115614. [Google Scholar] [CrossRef]
- Xu, M.; Hu, H.; Yang, Y.; Huang, Y.; Xie, K.; Liu, H.; Li, X.; Yao, H.; Naruse, I. A deep insight into carbon conversion during Zhundong coal molten salt gasification. Fuel 2018, 220, 890–897. [Google Scholar] [CrossRef]
- Singh, K.P.; Kakati, M.C. Comprehensive models for predicting aromaticity of coals. Chem. Eng. Commun. 2003, 190, 1335–1347. [Google Scholar] [CrossRef]
- Sakintuna, B.; Yürüm, Y.; Çetinkaya, S. Evolution of carbon microstructures during the pyrolysis of Turkish Elbistan Lignite in the temperature range 700–1000 °C. Energy Fuels 2004, 18, 883–888. [Google Scholar] [CrossRef]
- Samaras, P.; Diamadopoulos, E.; Sakellaropoulos, G.P. The effect of mineral matter and pyrolysis conditions on the gasification of Greek lignite by carbon dioxide. Fuel 1996, 75, 1108–1114. [Google Scholar] [CrossRef]
- Lampropoulos, A.; Kaklidis, N.; Athanasiou, C.; Montes-Morán, M.A.; Arenillas, A.; Menéndez, J.A.; Binas, V.D.; Konsolakis, M.; Marnellos, G.E. Effect of Olive Kernel thermal treatment (torrefaction vs. slow pyrolysis) on the physicochemical characteristics and the CO2 or H2O gasification performance of as-prepared biochars. Int. J. Hydrog. Energy 2020, 46, 29126–29141. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Y.; Lin, X.; Wang, G.; Zheng, P.; Yang, Y.; Mochida, I. Structure and CO2 Gasification Reactivity of Char Derived through Pressured Hydropyrolysis from Low-Rank Coal. Energy Fuels 2019, 33, 8032–8039. [Google Scholar] [CrossRef]
- Nawaz, S.; Butt, M.; Sheikh, N.; Hassan, M. Chemical, Microscopic and XRD Studies on Makerwal Coal. J. Fac. Eng. Technol. 2010, 16, 41–49. [Google Scholar]
- Zhu, X.; Sheng, C. Evolution of the char structure of lignite under heat treatment and its influences on combustion reactivity. Energy Fuels 2010, 24, 152–159. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Qi, J.; Fan, C.; Li, S. Characteristics of lignite char derived from oxy-pyrolysis. Fuel 2021, 291, 120261. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, G. Studying effects of solid structure evolution on gasification reactivity of coal chars by in-situ Raman spectroscopy. Fuel 2020, 270, 117603. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Liu, Z.; Ding, H.; Huang, X.; Zheng, C. Study on the evolution of the char structure during hydrogasification process using Raman spectroscopy. Fuel 2015, 157, 97–106. [Google Scholar] [CrossRef]
- Ma, C.; Zou, C.; Zhao, J.; Shi, R.; Li, X.; He, J.; Zhang, X. Pyrolysis Characteristics of Low-Rank Coal under a CO-Containing Atmosphere and Properties of the Prepared Coal Chars. Energy Fuels 2019, 33, 6098–6112. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Wang, Y.; Fu, Y.; Guo, Y.; Zhang, Y. Studies of low-temperature pyrolysis characteristics of the binder cold-briquetted lignite-II: Three-phase pyrolytic products. J. Energy Inst. 2017, 90, 776–786. [Google Scholar] [CrossRef]
- Wang, M.; Roberts, D.G.; Kochanek, M.A.; Harris, D.J.; Chang, L.; Li, C.Z. Raman spectroscopic investigations into links between intrinsic reactivity and char chemical structure. Energy Fuels 2014, 28, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Sheng, C. Influences of carbon structure on the reactivities of lignite char reacting with CO2 and NO. Fuel Process. Technol. 2010, 91, 837–842. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Sun, J.; Feng, H.; Xu, J.; Jin, H.; Guo, L. Investigation of the conversion mechanism for hydrogen production by coal gasification in supercritical water. Int. J. Hydrog. Energy 2021, 46, 10205–10215. [Google Scholar] [CrossRef]
- Gmbh, B. Review of the kinetics of pyrolysis and hydropyrolysis in relation to the chemical constitution of coal. Fuel 1983, 63, 731–737. [Google Scholar]
- Wang, L.; Alsaker, N.; Skreiberg, O.; Hovd, B. Effect of carbonization conditions on CO2 gasification reactivity of biocarbon. Energy Procedia 2017, 142, 932–937. [Google Scholar] [CrossRef]
- Hussein, A.; Larachi, F.; Ziegler, D.; Alamdari, H. Effects of heat treatment and acid washing on properties and reactivity of charcoal. Biomass Bioenergy 2016, 90, 101–113. [Google Scholar] [CrossRef]
- Kandasamy, J.; Gökalp, I. Pyrolysis and Gasification Characteristics of High Ash Indian and Turkish Coals. Gasif. Low-Grade Feed. 2018. [Google Scholar] [CrossRef] [Green Version]
- Shahabuddin, M.; Kibria, M.A.; Bhattacharya, S. Evaluation of high-temperature pyrolysis and CO2 gasification performance of bituminous coal in an entrained flow gasifier. J. Energy Inst. 2021, 94, 294–309. [Google Scholar] [CrossRef]
- Wei, R.; Ren, L.; Geng, F. Gasification reactivity and characteristics of coal chars and petcokes. J. Energy Inst. 2021, 96, 25–30. [Google Scholar] [CrossRef]
- Porada, S. The reactions of formation of selected gas products during coal pyrolysis. Fuel 2004, 83, 1191–1196. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Wang, Y.; Zhang, G.; Chen, L. Gas evolution characteristics of lignite during low-temperature pyrolysis. J. Anal. Appl. Pyrolysis 2013, 104, 625–631. [Google Scholar] [CrossRef]
- Gao, S.P.; Zhao, J.T.; Wang, Z.Q.; Wang, J.F.; Fang, Y.T.; Huang, J.J. Effect of CO2 on pyrolysis behaviors of lignite. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2013, 41, 257–264. [Google Scholar] [CrossRef]
- Abu-zahra, M.R.M.; Feron, P.H.M.; Versteeg, G.F. CO2 capture from power plants Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2011, 40, 2005. [Google Scholar] [CrossRef]
- Standish, N.; Tanjung, A.F.A. Gasification of single wood charcoal particles in CO2. Fuel 1988, 67, 666–672. [Google Scholar] [CrossRef]
- Kwon, T.W.; Kim, S.D.; Fung, D.P.C. Reaction kinetics of char-CO2 gasification. Fuel 1988, 67, 530–535. [Google Scholar] [CrossRef]
- Ahn, D.H.; Gibbs, B.M.; Ko, K.H.; Kim, J.J. Gasification kinetics of an Indonesian sub-bituminous coal-char with CO2 at elevated pressure. Fuel 2001, 80, 1651–1658. [Google Scholar] [CrossRef]
- Adánez, J.; Miranda, J.L.; Gavilán, J.M. Kinetics of a lignite-char gasification by CO2. Fuel 1985, 64, 801–804. [Google Scholar] [CrossRef]
- He, Q.; Guo, Q.; Ding, L.; Wei, J.; Yu, G. CO2 gasification of char from raw and torrefied biomass: Reactivity, kinetics and mechanism analysis. Bioresour. Technol. 2019, 293, 122087. [Google Scholar] [CrossRef]
- Ahmed, I.; Gupta, A.K. Characteristics of cardboard and paper gasification with CO2. Appl. Energy 2009, 86, 2626–2634. [Google Scholar] [CrossRef]
- Guizani, C. Effects of CO2 on the biomass pyro-gasification in High Heating Rate and Low Heating Rate conditions. Ph.D. Thesis, Ecole des Mines d’Albi-Carmaux, Albi, France, 2014; p. 277. [Google Scholar]
- Manera, C.; Perondi, D.; Barcellos, T.; Godinho, M. CO2 gasification of elephant grass in a fixed bed reactor. Sci. Cum. Ind. 2018, 6, 27–30. [Google Scholar] [CrossRef]
- Porada, S.; Czerski, G.; Grzywacz, P.; Makowska, D.; Dziok, T. Comparison of the gasification of coals and their chars with CO2 based on the formation kinetics of gaseous products. Thermochim. Acta 2017, 653, 97–105. [Google Scholar] [CrossRef]













| Fuel | LG | LG300 | LG500 | LG800 |
|---|---|---|---|---|
| Proximate analysis (wt.%) adb | ||||
| Fixed carbon | 17.96 | 19.12 | 21.89 | 25.23 |
| Volatile matter | 41.85 | 38.84 | 29.31 | 11.79 |
| Ash | 40.18 | 42.03 | 48.79 | 62.97 |
| Moisture | 7.67 | 3.36 | 2.82 | 0.91 |
| Elemental analysis (wt.%) db | ||||
| Carbon | 36.22 | 36.32 | 35.26 | 32.01 |
| Hydrogen | 2.94 | 2.75 | 1.75 | 1.01 |
| Sulfur | 0.97 | 0.92 | 0.68 | 0.90 |
| Nitrogen | 1.05 | 1.05 | 0.99 | 0.73 |
| Oxygen * | 18.63 | 16.93 | 12.53 | 0.37 |
| Aromaticity factor daf,a | 0.41 | 0.44 | 0.54 | 0.79 |
| Aromaticity factor b | 0.39 | 0.42 | 0.47 | 0.74 |
| Fuel | Devolatilization Stage | ||||
|---|---|---|---|---|---|
| Temp. Range, °C | Weight Loss, % | da/dtmax, min−1 | da/dtmean, min−1 | S × 10−12 | |
| LG | 150–650 | 22.6 | 0.022 | 0.014 | 1.85 |
| LG300 | 150–650 | 21.6 | 0.022 | 0.014 | 1.85 |
| LG500 | 150–650 | 13.8 | 0.012 | 0.009 | 0.68 |
| LG800 | 150–700 | 4 | 0.007 | 0.002 | 0.22 |
| Fuel | Gasification Stage | ||||
| Temp. Range,°C | Weight Loss, % | da/dtmax, min−1 | da/dtmean, min−1 | S × 10−12 | |
| LG | 650–920 | 29 | 0.06 | 0.04 | 2.24 |
| LG300 | 650–920 | 30 | 0.064 | 0.042 | 2.50 |
| LG500 | 650–920 | 31 | 0.076 | 0.051 | 3.69 |
| LG800 | 700–920 | 31 | 0.1 | 0.059 | 5.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampropoulos, A.; Binas, V.D.; Zouridi, L.; Athanasiou, C.; Montes-Morán, M.A.; Menéndez, J.A.; Konsolakis, M.; Marnellos, G.E. CO2 Gasification Reactivity and Syngas Production of Greek Lignite Coal and Ex-Situ Produced Chars under Non-Isothermal and Isothermal Conditions: Structure-Performance Relationships. Energies 2022, 15, 679. https://doi.org/10.3390/en15030679
Lampropoulos A, Binas VD, Zouridi L, Athanasiou C, Montes-Morán MA, Menéndez JA, Konsolakis M, Marnellos GE. CO2 Gasification Reactivity and Syngas Production of Greek Lignite Coal and Ex-Situ Produced Chars under Non-Isothermal and Isothermal Conditions: Structure-Performance Relationships. Energies. 2022; 15(3):679. https://doi.org/10.3390/en15030679
Chicago/Turabian StyleLampropoulos, Athanasios, Vassilios D. Binas, Leila Zouridi, Costas Athanasiou, Miguel A. Montes-Morán, J. Angel Menéndez, Michalis Konsolakis, and George E. Marnellos. 2022. "CO2 Gasification Reactivity and Syngas Production of Greek Lignite Coal and Ex-Situ Produced Chars under Non-Isothermal and Isothermal Conditions: Structure-Performance Relationships" Energies 15, no. 3: 679. https://doi.org/10.3390/en15030679
APA StyleLampropoulos, A., Binas, V. D., Zouridi, L., Athanasiou, C., Montes-Morán, M. A., Menéndez, J. A., Konsolakis, M., & Marnellos, G. E. (2022). CO2 Gasification Reactivity and Syngas Production of Greek Lignite Coal and Ex-Situ Produced Chars under Non-Isothermal and Isothermal Conditions: Structure-Performance Relationships. Energies, 15(3), 679. https://doi.org/10.3390/en15030679