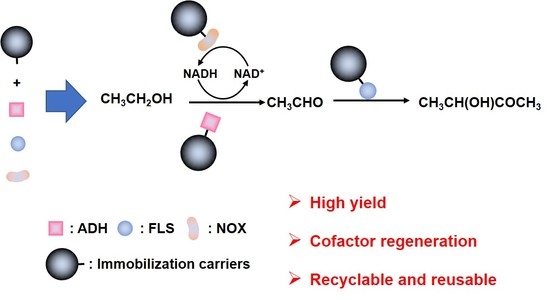
Immobilization of Alcohol Dehydrogenase, Acetaldehyde Lyase, and NADH Oxidase for Cascade Enzymatic Conversion of Ethanol to Acetoin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Enzyme Assays
2.3. Synthesis of Fe3O4@SiO2-Epoxy Nanoparticles
2.4. Expression and Purification of Recombinant Enzymes
2.5. Immobilization of Enzymes
2.6. The Synthesis of Acetoin with Immobilized Enzymes
2.7. The Reusability of Immobilized Cascade Enzymes
3. Results and Discussion
3.1. The Selection of Cascade Enzymes and the Enzyme Assays
3.2. The Effects of Temperature and pH on the Immobilization
3.3. The Effects of the Immobilization Time and the Enzyme Carrier Ratio on the Immobilization
3.4. The Synthesis of Acetoin with Immobilized Cascade Enzymes and Free Cascade Enzymes
3.5. The Reusability of Immobilized Cascade Enzymes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | Alcohol dehydrogenase |
| DTT | Dithiothreitol |
| FLS | Acetaldehyde lyase |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| NADH/NAD+ | Reduced nicotinamide adenine dinucleotide |
| NOX | NADH oxidase |
| TEOS | Tetraethyl orthosilicate |
| TPP | Thiamine pyrophosphate |
| Tris | Tris(hydroxymethyl)methyl aminomethane |
References
- Zhang, Q.; Yang, X.; Guan, J. Applications of magnetic nanomaterials in heterogeneous catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sarp, G.; Uzcan, F.; Ozalp, O.; Soylak, M. Application of magnetic nanomaterials in bioanalysis. Talanta 2021, 229, 122285. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.-Y.; Jiang, X.-P.; Ye, J.-J.; Zhang, Y.-W.; Zhang, X.-Y. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulase. J. Nanopart. Res 2015, 17, 8. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jiang, X.-P.; Li, Y.; Zeng, S.; Zhang, Y.-W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lu, J.R. Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, X.; Yu, W.; Wen, Z.; Chen, S. Enhancement of acetoin production from bacillus licheniformis by 2,3-butanediol conversion strategy: Metabolic engineering and fermentation control. Process Biochem. 2017, 57, 35–42. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S.-T. Metabolic engineering strategies for acetoin and 2,3-butanediol production: Advances and prospects. Crit. Rev. Biotechnol. 2017, 37, 990–1005. [Google Scholar] [CrossRef]
- Peng, K.; Guo, D.; Lou, Q.; Lu, X.; Cheng, J.; Qiao, J.; Lu, L.; Cai, T.; Liu, Y.; Jiang, H. synthesis of ligustrazine from acetaldehyde by a combined biological–chemical approach. ACS Synth. Biol. 2020, 9, 2902–2908. [Google Scholar] [CrossRef]
- Wang, X.; Lv, M.; Zhang, L.; Li, K.; Gao, C.; Ma, C.; Xu, P. Efficient bioconversion of 2,3-butanediol into acetoin using gluconobacter oxydans DSM 2003. Biotechnol. Biofuels 2013, 6, 155. [Google Scholar] [CrossRef] [Green Version]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 55, 107783. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; DOE/GO-102004-1992; National Renewable Energy Laboratory: Golden, CO, USA, 2004; p. 15008859. [Google Scholar]
- Lü, C.; Ge, Y.; Cao, M.; Guo, X.; Liu, P.; Gao, C.; Xu, P.; Ma, C. Metabolic engineering of bacillus licheniformis for production of acetoin. Front. Bioeng. Biotechnol. 2020, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, J.; Gao, C.; Hua, D.; Ma, C.; Li, L.; Wang, Y.; Xu, P. Production of (2S,3S)-2,3-butanediol and (3S)-acetoin from glucose using resting cells of Klebsiella pneumonia and Bacillus subtilis. Bioresour. Technol. 2011, 102, 10741–10744. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-A.; Zhang, L.-Y.; Rao, B.; Shen, Y.-L.; Wei, D.-Z. Enhanced acetoin production by serratia marcescens H32 with expression of a water-forming nadh oxidase. Bioresour. Technol. 2012, 119, 94–98. [Google Scholar] [CrossRef]
- Wang, M.; Fu, J.; Zhang, X.; Chen, T. Metabolic engineering of bacillus subtilis for enhanced production of acetoin. Biotechnol. Lett. 2012, 34, 1877–1885. [Google Scholar] [CrossRef]
- Lu, L.; Mao, Y.; Kou, M.; Cui, Z.; Jin, B.; Chang, Z.; Wang, Z.; Ma, H.; Chen, T. Engineering Central pathways for industrial-level (3r)-acetoin biosynthesis in corynebacterium glutamicum. Microb. Cell Factories 2020, 19, 102. [Google Scholar] [CrossRef]
- Bae, S.-J.; Kim, S.; Hahn, J.-S. Efficient production of acetoin in saccharomyces cerevisiae by disruption of 2,3-butanediol dehydrogenase and expression of NADH oxidase. Sci. Rep. 2016, 6, 27667. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Wu, Y.; Yang, L.; Wang, Z.; Chen, T. Engineering genome-reduced Bacillus subtilis for acetoin production from xylose. Biotechnol. Lett. 2018, 40, 393–398. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Z.; Zheng, M.; Chen, T. Advances in biological production of acetoin: A comprehensive overview. Crit. Rev. Biotechnol. 2021, 93, 1–22. [Google Scholar] [CrossRef]
- Zhang, L.; Singh, R.D.S.; Guo, Z.; Li, J.; Chen, F.; He, Y.; Guan, X.; Kang, Y.C.; Lee, J.-K. An artificial synthetic pathway for acetoin, 2,3-butanediol, and 2-butanol production from ethanol using cell free multi-enzyme catalysis. Green Chem. 2018, 20, 230–242. [Google Scholar] [CrossRef]
- Hohagen, H.; Schwarz, D.; Schenk, G.; Guddat, L.W.; Schieder, D.; Carsten, J.; Sieber, V. Deacidification of grass silage press juice by continuous production of acetoin from its lactate via an immobilized enzymatic reaction cascade. Bioresour. Technol. 2017, 245, 1084–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-P.; Lu, T.-T.; Liu, C.-H.; Ling, X.-M.; Zhuang, M.-Y.; Zhang, J.-X.; Zhang, Y.-W. Immobilization of dehydrogenase onto epoxy-functionalized nanoparticles for synthesis of (R)-mandelic acid. Int. J. Biol. Macromol. 2016, 88, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Shi, Y.; Zhang, J.-X.; Gao, J.; Zhang, Y.-W. Cloning, expression, characterization and homology modeling of a novel water-forming NADH oxidase from Streptococcus mutans ATCC 25175. Int. J. Biol. Macromol. 2018, 113, 1073–1079. [Google Scholar] [CrossRef]
- Behling, R.; Valange, S.; Chatel, G. Heterogeneous Catalytic oxidation for lignin valorization into valuable chemicals: What results? What limitations? What trends? Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Siegel, J.B.; Smith, A.L.; Poust, S.; Wargacki, A.J.; Bar-Even, A.; Louw, C.; Shen, B.W.; Eiben, C.B.; Tran, H.M.; Noor, E.; et al. Computational protein design enables a novel one-carbon assimilation pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 3704–3709. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhuang, M.-Y.; Jiang, X.-P.; Ling, X.-M.; Xu, M.-Q.; Zhu, Y.-H.; Zhang, Y.-W. Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration: Enzymatic production of 1, 3-dihydroxyacetone via immobilized enzymes. J. Chem. Technol. Biotechnol. 2018, 93, 2351–2358. [Google Scholar] [CrossRef]
- Nisar, M.A.; Rashid, N.; Bashir, Q.; Gardner, Q.-A.A.; Shafiq, M.H.; Akhtar, M. TK1299, a highly thermostable NAD(P)H oxidase from Thermococcus kodakaraensis exhibiting higher enzymatic activity with NADPH. J. Biosci. Bioeng. 2013, 116, 39–44. [Google Scholar] [CrossRef]
- Palma-Gutiãrez, H.N.; Rodrãguez-Zavala, J.S.; Jasso-Chãvez, R.; Moreno-Sãnchez, R.; Saavedra, E. Gene Cloning and Biochemical Characterization of an Alcohol Dehydrogenase from Euglena gracilis. J. Eukaryot. Microbiol. 2008, 55, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Tiwari, M.K.; Kang, Y.C.; Lee, J.-K. Characterization of H2O-forming NADH Oxidase from Streptococcus pyogenes and its application in l-rare sugar production. Bioorganic Med. Chem. Lett. 2012, 22, 1931–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Tiwari, M.K.; Gao, H.; Dhiman, S.S.; Jeya, M.; Lee, J.-K. Cloning and characterization of a thermostable H2O-forming NADH oxidase from Lactobacillus rhamnosus. Enzym. Microb. Technol. 2012, 50, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Su, W.B.; Tao, Q.-L.; Zhang, L.-Y.; Zhang, Y.-W. Expression, biochemical characterization, and mutation of a water forming NADH: FMN oxidoreductase from Lactobacillus rhamnosus. Enzym. Microb. Technol. 2020, 134, 109464. [Google Scholar] [CrossRef]
- Kawasaki, S.; Ishikura, J.; Chiba, D.; Nishino, T.; Niimura, Y. Purification and characterization of an H2O-forming NADH oxidase from Clostridium aminovalericum: Existence of an oxygen-detoxifying enzyme in an obligate anaerobic bacteria. Arch. Microbiol. 2004, 181, 324–330. [Google Scholar] [CrossRef]
- Ma, M.; Wang, X.; Zhang, X.; Zhao, X. Alcohol dehydrogenases from scheffersomyces stipitis involved in the detoxification of aldehyde inhibitors derived from lignocellulosic biomass conversion. Appl. Microbiol. Biotechnol. 2013, 97, 8411–8425. [Google Scholar] [CrossRef]
- Nie, Y.; Xu, Y.; Mu, X.Q.; Wang, H.Y.; Yang, M.; Xiao, R. Purification, characterization, gene cloning, and expression of a novel alcohol dehydrogenase with anti-prelog stereospecificity from Candida parapsilosis. Appl. Environ. Microbiol. 2007, 73, 3759–3764. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Liao, L.; Xu, X.; Oren, A.; Wang, C.; Zhu, X.; Wu, M. Characterization of alcohol dehydrogenase from the Haloalkaliphilic Archaeon Natronomonas pharaonis. Extremophiles 2008, 12, 471–476. [Google Scholar] [CrossRef]
- Timpson, L.M.; Alsafadi, D.; Mac Donnchadha, C.; Liddell, S.; Sharkey, M.A.; Paradisi, F. Characterization of alcohol dehydrogenase (ADH12) from Haloarcula marismortui, an Extreme halophile from the dead sea. Extremophiles 2012, 16, 57–66. [Google Scholar] [CrossRef]
- De María, P.D.; Stillger, T.; Pohl, M.; Wallert, S.; Drauz, K.; Gröger, H.; Trauthwein, H.; Liese, A. Preparative enantioselective synthesis of benzoins and (r)-2-hydroxy-1-phenylpropanone using benzaldehyde lyase. J. Mol. Catal. B Enzym. 2006, 38, 43–47. [Google Scholar] [CrossRef]
- Fraenkel-Conrat, H. The action of 1,2-epoxides on proteins. J. Biol. Chem. 1944, 154, 227–238. [Google Scholar] [CrossRef]
- Bearne, S.L. Illustrating the effect of pH on enzyme activity using gibbs energy profiles. J. Chem. Educ. 2014, 91, 84–90. [Google Scholar] [CrossRef]
- Chaloupkova, R.; Prokop, Z.; Sato, Y.; Nagata, Y.; Damborsky, J. Stereoselectivity and conformational stability of haloalkane dehalogenase dbja from Bradyrhizobium japonicum USDA110: The effect of pH and temperature: Stereochemistry and conformational stability of DbjA. FEBS J. 2011, 278, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Q.; Li, F.-L.; Yu, W.-Q.; Li, R.-F.; Zhang, Y.-W. Combined cross-linked enzyme aggregates of glycerol dehydrogenase and NADH oxidase for high efficiency in situ NAD+ regeneration. Int. J. Biol. Macromol. 2020, 144, 1013–1021. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Huber, C.; Nidetzky, B.; Bolivar, J.M.; López-Gallego, F. Design of the enzyme–carrier interface to overcome the O2 and NADH mass transfer limitations of an immobilized flavin oxidase. ACS Appl. Mater. Interfaces 2020, 12, 56027–56038. [Google Scholar] [CrossRef]
- Cui, Z.; Mao, Y.; Zhao, Y.; Zheng, M.; Wang, Z.; Ma, H.; Chen, T. One-Pot efficient biosynthesis of (3R)-acetoin from pyruvate by a Two-Enzyme cascade. Catal. Sci. Technol. 2020, 10, 7734–7744. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, Y.; Mao, Y.; Shi, T.; Lu, L.; Ma, H.; Wang, Z.; Chen, T. In Vitro biosynthesis of optically pure D—(−)—Acetoin from meso-2,3-butanediol using 2,3-butanediol dehydrogenase and NADH oxidase. J. Chem. Technol. Biotechnol. 2019, 94, 2547–2554. [Google Scholar] [CrossRef]






| Enzyme | Organism | Vmax (U·mg−1) | Km (µM) | Topt (°C) | pHopt | Reference |
|---|---|---|---|---|---|---|
| NOX | Lactobacillus Rhamnosus | 263 | 5.8 | 50 | 5.5 | [34] |
| Lactobacillus rhamnosus | NA | 24 | 40 | 6.0 | [35] | |
| Clostridium Aminovalericum | 119 | 19.2 | 40 | 6.0 | [36] | |
| Streptococcus Mutans | 154.3 | 57.7 | 35 | 7.0 | [26] | |
| Streptococcus Pyogenes | 344 | 27.0 | 55 | 7.0 | [33] | |
| Thermococcus Kodakaraensis | 83.1 | 24.2 | 75 | 8.0 | [31] | |
| ADH | Euglena Gracilis | 11.7 | 3.2 | NA | 7.0 | [32] |
| NA | 10.64 | 0.37 | 25 | 8.0 | [27] | |
| Lignocellulosic | 4.33 | 3.15 | 30 | 6.0 | [37] | |
| Candida Parapsilosis | NA | NA | 35 | 4.5 | [38] | |
| Natronomonas pharaonis | 0.36 | 3.4 × 105 | 70 | 9.0 | [39] | |
| Haloarcula Marismortui | 1.6 | 5.1 | 60 | NA | [40] | |
| FLS | Pseudomonas fluorescens | NA | NA | NA | 9.5 | [41] |
| Pseudomonas fluorescens | NA | NA | 35 | NA | [9] | |
| Pseudomonas fluorescens | NA | NA | NA | 8.0 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-Y.; Huang, J.-Y.; Zhou, Q.; Xu, Y.-Y.; Prabhu, P.; Zhang, Y.-W. Immobilization of Alcohol Dehydrogenase, Acetaldehyde Lyase, and NADH Oxidase for Cascade Enzymatic Conversion of Ethanol to Acetoin. Energies 2022, 15, 4242. https://doi.org/10.3390/en15124242
Li X-Y, Huang J-Y, Zhou Q, Xu Y-Y, Prabhu P, Zhang Y-W. Immobilization of Alcohol Dehydrogenase, Acetaldehyde Lyase, and NADH Oxidase for Cascade Enzymatic Conversion of Ethanol to Acetoin. Energies. 2022; 15(12):4242. https://doi.org/10.3390/en15124242
Chicago/Turabian StyleLi, Xue-Yong, Jia-Ying Huang, Qiang Zhou, Yuan-Yuan Xu, Ponnandy Prabhu, and Ye-Wang Zhang. 2022. "Immobilization of Alcohol Dehydrogenase, Acetaldehyde Lyase, and NADH Oxidase for Cascade Enzymatic Conversion of Ethanol to Acetoin" Energies 15, no. 12: 4242. https://doi.org/10.3390/en15124242
APA StyleLi, X.-Y., Huang, J.-Y., Zhou, Q., Xu, Y.-Y., Prabhu, P., & Zhang, Y.-W. (2022). Immobilization of Alcohol Dehydrogenase, Acetaldehyde Lyase, and NADH Oxidase for Cascade Enzymatic Conversion of Ethanol to Acetoin. Energies, 15(12), 4242. https://doi.org/10.3390/en15124242