Ethanol-Assisted Hydrothermal Liquefaction of Poplar Using Fe-Co/Al2O3 as Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst-Preparation Procedure
2.3. Experimental Procedure
2.4. Catalyst- and Product-Characterization Method
3. Results and Discussion
3.1. Catalyst- and Product-Characterization Method
3.2. Effects of Solvents at Different Temperatures
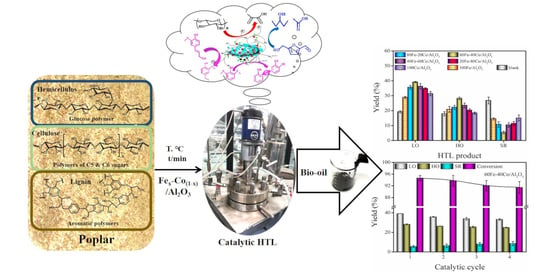
3.3. Experimental Results of Catalytic Hydrothermal Liquefaction
3.3.1. Effect of Temperature
3.3.2. Effect of Fe and Co Content
3.3.3. Effect of Residence Time
3.4. Bio-Oil Analysis
3.4.1. GC-MS Analysis and Mechanism of Catalytic Liquefaction
3.4.2. HHV and Energy Recovery
3.4.3. FT-IR Analysis
3.4.4. TGA Analysis
3.5. Catalyst Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404–1446. [Google Scholar] [CrossRef]
- Ghimire, N.; Bakke, R.; Bergland, W.H. Liquefaction of lignocellulosic biomass for methane production: A review. Bioresour. Technol. 2021, 332, 125068. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, B.N.; Baryshnikov, S.V.; Malyar, Y.N.; Skripnikov, A.M.; Taran, O.P. Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst. Catalysts 2021, 11, 1362. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.H.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Zhu, S.; Tian, F.; Xu, Y.; Zhu, C.; Dong, L. Synergistic hydrothermal liquefaction of wheat stalk with homogeneous and heterogeneous catalyst at low temperature. Bioresour. Technol. 2019, 278, 92–98. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Qi, L.; Gao, J.; Xu, C.C. Promotion effects of metallic iron on hydrothermal liquefaction of cornstalk in ethanol-water mixed solvents for the production of biocrude oil. Fuel 2021, 285, 119150. [Google Scholar] [CrossRef]
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Roles of Co-solvents in Hydrothermal Liquefaction of Low-Lipid, High-Protein Algae. Bioresour. Technol. 2020, 310, 123454. [Google Scholar] [CrossRef]
- Cheng, S.; Wilks, C.; Yuan, Z.; Leitch, M.; Xu, C.C. Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water-ethanol co-solvent. Polym. Degrad. Stabil. 2012, 97, 839–848. [Google Scholar] [CrossRef]
- Lai, F.; Chang, Y.; Huang, H.; Wu, G.; Xiong, J.; Pan, Z.; Zhou, C. Liquefaction of sewage sludge in ethanol-water mixed solvents for bio-oil and biochar products. Energy 2018, 148, 629–641. [Google Scholar] [CrossRef]
- Durak, H. Bio-oil production from Glycyrrhiza glabra through supercritical fluid extraction. J. Supercrit. Fluids 2014, 95, 373–386. [Google Scholar] [CrossRef]
- Remón, J.; Randall, J.; Budarin, V.L.; Clark, J.H. Production of bio-fuels and chemicals by microwave-assisted, catalytic, hydrothermal liquefaction (MAC-HTL) of a mixture of pine and spruce biomass. Green Chem. 2019, 21, 284–299. [Google Scholar] [CrossRef]
- Cao, B.; Yuan, J.; Jiang, D.; Wang, S.; Wang, Q. Seaweed-derived biochar with multiple active sites as a heterogeneous catalyst for converting macroalgae into acid-free biooil containing abundant ester and sugar substances. Fuel 2020, 285, 119164. [Google Scholar] [CrossRef]
- Wang, S. High-Efficiency Separation of Bio-Oil. In Biomass Now: Sustainable Growth and Use; IntechOpen: Rijeka, Croatia, 2013; p. 81246. [Google Scholar] [CrossRef] [Green Version]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Huang, H. Nitrogen in bio-oil produced from hydrothermal liquefaction of biomass: A review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Jiang, J.; Yang, Z.; Feng, W.; Li, L.; Guo, Y.; Hu, J. Catalytic hydrothermal liquefaction of Gracilaria corticata macroalgae: Effects of process parameter on bio-oil up-gradation. Bioresour. Technol. 2020, 319, 124163. [Google Scholar] [CrossRef]
- Wang, B.; He, Z.; Zhang, B.; Duan, Y. Study on hydrothermal liquefaction of spirulina platensis using biochar based catalysts to produce bio-oil. Energy 2021, 230, 120733. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.A.; Bisht, Y.; Krishna, B.B.; Bhaskar, T. Role of temperatures and solvents on hydrothermal liquefaction of Azolla filiculoides. Energy 2020, 217, 119330. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagata, K.; Hirose, M.; Yamazaki, Y.; Nishimura, A.; Okuda, N.; Arita, Y.; Hirano, Y.; Kita, Y. Fe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass for Producing High-Grade Bio-Oil. ACS Sustain. Chem. Eng. 2017, 5, 3562–3569. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Le, T.M.; Nguyen, H.V. Iron-catalyzed fast hydrothermal liquefaction of Cladophora socialis macroalgae into high quality fuel precursor. Bioresour. Technol. 2021, 337, 125445. [Google Scholar] [CrossRef]
- Xu, J.; Dong, X.; Wang, Y. Hydrothermal liquefaction of macroalgae over various solids, basic or acidic oxides and metal salt catalyst: Products distribution and characterization. Ind. Crops Prod. 2020, 151, 112458. [Google Scholar] [CrossRef]
- Caprariis, B.; Filippis, P.D.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent Advances in Catalytic Conversion of Ethanol to Chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Devi, T.E.; Parthiban, R. Hydrothermal liquefaction of Nostoc ellipsosporum biomass grown in municipal wastewater under optimized conditions for bio-oil production. Bioresour. Technol. 2020, 316, 123943. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, X.; Wang, Z.; Liu, B. Catalytic hydrothermal liquefaction of lignin for production of aromatic hydrocarbon over metal supported mesoporous catalyst. Bioresour. Technol. 2021, 323, 124569. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.; Chen, W.; Schideman, L.C.; Leon, R. Effects of reaction temperature and reaction time on the hydrothermal liquefaction of demineralized wastewater algal biomass. Bioresour. Technol. Rep. 2021, 14, 100679. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Highly efficient catalytic pyrolysis of biomass vapors upgraded into jet fuel range hydrocarbonrich bio-oil over a bimetallic Pt-Ni/g-Al2O3 catalyst. Int. J. Hydrogen Energy 2021, 46, 27922–27940. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S. Hydrothermal liquefaction of lignocellulosic biomass using potassium fluoride-doped alumina. Energy Fuel 2019, 33, 3248–3256. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Wang, H.; Hu, Y.; Xu, C.C. Synergistic effects of metallic Fe and other homogeneous/heterogeneous catalysts in hydrothermal liquefaction of woody biomass. Renew. Energy 2021, 176, 543–554. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Rabnawaz, M. Upgrading pyrolysis bio-oil through hydrodeoxygenation (HDO) using non-sulfided Fe-Co/SiO2 catalyst. Energy Convers. Manag. 2017, 150, 331–342. [Google Scholar] [CrossRef]
- Vo, T.K.; Kim, S.S.; Ly, H.V.; Lee, E.Y.; Lee, C.G.; Kim, J. A general reaction network and kinetic model of the hydrothermal liquefaction of microalgae Tetraselmis sp. Bioresour. Technol. 2017, 241, 610–619. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S. Catalytic hydrothermal liquefaction of lactuca scariola with a heterogeneous catalyst: The investigation of temperature, reaction time and synergistic effect of catalysts. Bioresour. Technol. 2020, 309, 123375. [Google Scholar] [CrossRef]
- Kumar, A.; Biawas, B.; Bhaskar, T. Effect of cobalt on titania, ceria and zirconia oxide supported catalysts on the oxidative depolymerization of prot and alkali lignin. Bioresour. Technol. 2020, 299, 122589. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2021, 11, 42. [Google Scholar] [CrossRef]
- Hao, B.; Xu, D.; Jiang, G.; Sabri, T.A.; Jing, Z.; Guo, Y. Chemical reactions in the hydrothermal liquefaction of biomass and in the catalytic hydrogenation upgrading of biocrude. Green Chem. 2021, 23, 1562–1583. [Google Scholar] [CrossRef]
- Lu, Q.; Yuan, S.; Liu, C.; Zhang, T.; Xie, X.; Deng, X.; He, R. A Fe-Ca/SiO2 catalyst for efficient production of light aromatics from catalytic pyrolysis of biomass. Fuel 2020, 279, 118500. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Wallace, C.W.; Wang, L.; Dan, C. Enhanced bio-oil production from swine manure co-liquefaction with crude glycerol. Energy Convers. Manag. 2011, 52, 1004–1009. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Yuan, X.; Li, J.; Zhou, W. Beneficial synergistic effect on bio-oil production from co-liquefaction of sewage sludge and lignocellulosic biomass. Bioresour. Technol. 2017, 251, 49–56. [Google Scholar] [CrossRef]
- Malins, K. Production of bio-oil via hydrothermal liquefaction of birch sawdust. Energy Convers. Manag. 2017, 144, 243–251. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; Li, W.; Xu, C.C. Alkali-catalyzed liquefaction of pinewood sawdust in ethanol/water co-solvents. Biomass Bioenergy 2020, 134, 105485. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X.; Li, B.; Xiao, Y.; Zeng, G. Thermochemical liquefaction characteristics of sewage sludge in different organic solvents. J. Anal. Appl. Pyrolysis 2014, 109, 176–184. [Google Scholar] [CrossRef]










| Catalyst | S-Micro (m2/g) | Pore Volume (cm3/g) | V-Micro (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| 100Fe/Al2O3 | 59 | 0.21 | 0.03 | 17.2 |
| 80Fe-20Co/Al2O3 | 76 | 0.30 | 0.04 | 15.3 |
| 60Fe-40Co/Al2O3 | 85 | 0.30 | 0.05 | 14.7 |
| 40Fe-60Co/Al2O3 | 118 | 0.40 | 0.06 | 12.4 |
| 20Fe-80Co/Al2O3 | 123 | 0.44 | 0.06 | 11.6 |
| 100Co/Al2O3 | 99 | 0.31 | 0.05 | 13.8 |
| T/°C | Time/min | Catalyst | HHVLO (MJ/Kg) | ERRLO (%) | HHVHO (MJ/Kg) | ERRHO (%) | ERRTotal bio-oil (%) |
|---|---|---|---|---|---|---|---|
| 220 | 30 | / | 16.59 | 15.90 | 22.92 | 8.35 | 24.25 |
| 60Fe-40Co/Al2O3 | 15.47 | 21.78 | 25.64 | 18.30 | 40.08 | ||
| 260 | 10 | / | 17.33 | 19.04 | 25.21 | 10.62 | 29.66 |
| 60Fe-40Co/Al2O3 | 16.77 | 38.88 | 27.79 | 24.78 | 63.66 | ||
| 30 | / | 18.21 | 23.45 | 23.15 | 27.66 | 51.11 | |
| 100Fe/Al2O3 | 17.19 | 33.13 | 28.71 | 40.01 | 73.14 | ||
| 80Fe-20Co/Al2O3 | 16.52 | 39.35 | 27.96 | 39.21 | 78.56 | ||
| 60Fe-40Co/Al2O3 | 16.03 | 41.95 | 27.37 | 51.57 | 93.52 | ||
| 40Fe-60Co/Al2O3 | 15.83 | 38.41 | 27.12 | 41.88 | 80.29 | ||
| 20Fe-80Co/Al2O3 | 14.87 | 34.67 | 26.97 | 36.74 | 71.41 | ||
| 100Co/Al2O3 | 14.79 | 31.00 | 26.38 | 32.40 | 63.40 | ||
| 60 | / | 17.02 | 20.98 | 24.37 | 30.06 | 51.04 | |
| 60Fe-40Co/Al2O3 | 15.12 | 34.60 | 27.91 | 50.77 | 85.37 | ||
| 300 | 30 | / | 15.71 | 19.84 | 24.81 | 36.07 | 55.91 |
| 60Fe-40Co/Al2O3 | 14.85 | 26.79 | 27.87 | 51.64 | 78.23 |
| Sample | Distillate Range (°C) | Weight Loss Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Blank | 100Fe/Al2O3 | 80Fe-20Co/Al2O3 | 60Fe-40Co/Al2O3 | 40Fe-60Co/Al2O3 | 20Fe-80Co/Al2O3 | 100Co/Al2O3 | ||
| LO | 25–110 | 0.87 | 2.23 | 1.63 | 1.31 | 1.26 | 2.93 | 4.58 |
| 110–200 | 10.75 | 14.62 | 8.59 | 9.42 | 7.97 | 17.02 | 21.21 | |
| 200–300 | 31.42 | 28.18 | 28.81 | 34.71 | 29.08 | 28.36 | 24.13 | |
| 300–400 | 10.64 | 12.9 | 13.36 | 12.46 | 15.24 | 14.96 | 14.17 | |
| 400–550 | 8.02 | 7.81 | 6.92 | 7.35 | 9.52 | 7.89 | 7.30 | |
| 550–700 | 3.63 | 1.49 | 5.69 | 3.21 | 3.70 | 2.91 | 2.91 | |
| 700–800 | 3.42 | 1.64 | 2.45 | 2.70 | 1.30 | 1.54 | 1.39 | |
| >800 | 31.25 | 31.13 | 32.55 | 29.06 | 31.93 | 24.39 | 24.31 | |
| HO | 25–110 | 4.01 | 1.44 | 4.53 | 1.81 | 1.73 | 2.33 | 6.44 |
| 110–200 | 7.18 | 6.79 | 8.97 | 8.96 | 7.92 | 3.80 | 8.11 | |
| 200–300 | 11.87 | 12.10 | 13.47 | 13.70 | 14.10 | 14.70 | 14.56 | |
| 300–400 | 29.42 | 32.76 | 31.39 | 33.51 | 36.98 | 37.71 | 27.86 | |
| 400–550 | 10.93 | 14.13 | 10.6 | 13.85 | 15.31 | 16.44 | 8.61 | |
| 550–700 | 2.48 | 2.87 | 2.56 | 3.28 | 2.95 | 3.16 | 2.46 | |
| 700–800 | 1.03 | 1.01 | 1.15 | 1.26 | 1.02 | 1.12 | 1.04 | |
| >800 | 33.08 | 28.91 | 27.33 | 23.63 | 19.99 | 20.74 | 30.82 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Shakeel, U.; Zhang, Q.; Zhang, K.; Xu, X.; Xu, J. Ethanol-Assisted Hydrothermal Liquefaction of Poplar Using Fe-Co/Al2O3 as Catalyst. Energies 2022, 15, 3057. https://doi.org/10.3390/en15093057
Wu H, Shakeel U, Zhang Q, Zhang K, Xu X, Xu J. Ethanol-Assisted Hydrothermal Liquefaction of Poplar Using Fe-Co/Al2O3 as Catalyst. Energies. 2022; 15(9):3057. https://doi.org/10.3390/en15093057
Chicago/Turabian StyleWu, Haijun, Usama Shakeel, Quan Zhang, Kai Zhang, Xia Xu, and Jian Xu. 2022. "Ethanol-Assisted Hydrothermal Liquefaction of Poplar Using Fe-Co/Al2O3 as Catalyst" Energies 15, no. 9: 3057. https://doi.org/10.3390/en15093057
APA StyleWu, H., Shakeel, U., Zhang, Q., Zhang, K., Xu, X., & Xu, J. (2022). Ethanol-Assisted Hydrothermal Liquefaction of Poplar Using Fe-Co/Al2O3 as Catalyst. Energies, 15(9), 3057. https://doi.org/10.3390/en15093057