Free-Standing Li4Ti5O12/Carbon Nanotube Electrodes for Flexible Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Materials and MWCNT
2.2. Fabrication of Electrodes
2.3. Characterization Analyses
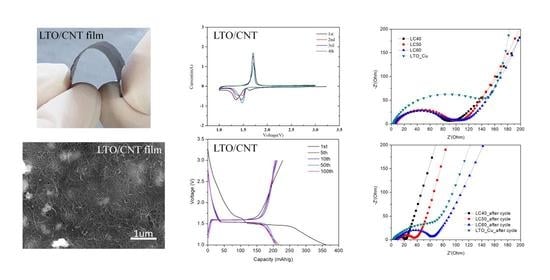
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qi, W.; Shapter, J.G.; Wu, Q.; Yin, T.; Gao, G.; Cui, D. Nanostructured anode materials for lithium-ion batteries: Principle, recent progress and future perspectives. J. Mater. Chem. A 2017, 5, 19521–19540. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.; White, R.E.; Doyle, M. Capacity Fade Mechanisms and Side Reactions in Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 3647–3667. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Mao, J. Enhanced the electrochemical performance of Li4Ti5O12 anode materials by high conductive graphene nanosheets. J. Mater. Sci. Mater. Electron. 2017, 28, 15135–15141. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Xu, B.; Lan, J.; Yu, Y.; Yang, X.; Lin, Y.; Nan, C. Li4Ti5O12 nanosheets assembled in tubular architecture for lithium storage. Chem. Eng. J. 2019, 361, 1371–1380. [Google Scholar] [CrossRef]
- Xu, G.; Han, P.; Dong, S.; Liu, H.; Cui, G.; Chen, L. Li4Ti5O12-based energy conversion and storage systems: Status and prospects. Coord. Chem. Rev. 2017, 343, 139–184. [Google Scholar] [CrossRef]
- Khan, F.; Oh, M.; Kim, J.H. N-functionalized graphene quantum dots: Charge transporting layer for high-rate and durable Li4Ti5O12-based Li-ion battery. Chem. Eng. J. 2019, 369, 1024–1033. [Google Scholar] [CrossRef]
- Kang, J.; Dong, G.; Li, Z.; Li, L. Preparation and electrochemical properties of nanorods and nanosheets structural Li4Ti5O12 as anode for lithium ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 12615–12623. [Google Scholar] [CrossRef]
- Lee, B.-G.; Ahn, H.-J.; Yoon, J.-R. Effects of post-calcination and mechanical pulverization on the electrochemical properties of nano-sized Li 4 Ti 5 O 12 for hybrid capacitors. Curr. Appl. Phys. 2017, 17, 121–125. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A comprehensive review of Li 4 Ti 5 O 12 -based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R. Rep. Rev. J. 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Hou, L.; Qin, X.; Gao, X.; Guo, T.; Li, X.; Li, J. Zr-doped Li4Ti5O12 anode materials with high specific capacity for lithium-ion batteries. J. Alloys Compd. 2019, 774, 38–45. [Google Scholar] [CrossRef]
- Reza, C.; Hernowo, M.F.; Syahrial, A.Z.; Subhan, A.; Priyono, B. Improved Li4Ti5O12performance with addition of graphite and Sn nanoparticles using the solid-state method as half-cell lithium-ion battery anode. AIP Conf. Proc. 2020, 2232, 030004. [Google Scholar] [CrossRef]
- Ncube, N.M.; Mhlongo, W.T.; McCrindle, R.I.; Zheng, H. The electrochemical effect of Al-doping on Li4Ti5O12 as anode material for lithium-ion batteries. Mater. Today Proc. 2018, 5, 10592–10601. [Google Scholar] [CrossRef]
- Lu, H.; Hagberg, J.; Lindbergh, G.; Cornell, A. Li4Ti5O12 flexible, lightweight electrodes based on cellulose nanofibrils as binder and carbon fibers as current collectors for Li-ion batteries. Nano Energy 2017, 39, 140–150. [Google Scholar] [CrossRef]
- Cho, H.; Son, H.; Kim, D.; Lee, M.; Boateng, S.; Han, H.S.; Kim, K.M.; Kim, S.; Choi, H.; Song, T.; et al. Impact of Mg-Doping Site Control in the Performance of Li4Ti5O12 Li-Ion Battery Anode: First-Principles Predictions and Experimental Verifications. J. Phys. Chem. C 2017, 121, 14994–15001. [Google Scholar] [CrossRef]
- Ye, Z.; Zhong, F.; Chen, Y.; Zou, Z.; Jiang, C. Unique CNTs-chained Li4Ti5O12 nanoparticles as excellent high rate anode materials for Li-ion capacitors. Ceram. Int. 2022, 48, 20237–20244. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, S.; Lian, Q.; Zhao, J.; Ding, W.; Yu, Z.; Huang, R.; Zou, Z. Nitrogen-doped carbon-coated hierarchical Li4Ti5O12-TiO2 hybrid microspheres as excellent high rate anode of Li-ion battery. Ceram. Int. 2017, 43, 11354–11360. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, J.-Y. One-pot sol-gel synthesis of Li 4 Ti 5 O 12/C anode materials for high-performance Li-ion batteries. Electrochim. Acta 2014, 142, 43–50. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008. [Google Scholar] [CrossRef]
- Aziz, F.; Ismail, A.F. Spray coating methods for polymer solar cells fabrication: A review. Mater. Sci. Semicond. Process. 2015, 39, 416–425. [Google Scholar] [CrossRef]
- Piffet, C.; Vertruyen, B.; Caes, S.; Thomassin, J.-M.; Broze, G.; Malherbe, C.; Boschini, F.; Cloots, R.; Mahmoud, A. Aqueous processing of flexible, free-standing Li4Ti5O12 electrodes for Li-ion batteries. Chem. Eng. J. 2020, 397, 125508. [Google Scholar] [CrossRef]
- Nyamaa, O.; Seo, D.-H.; Lee, J.-S.; Jeong, H.-M.; Huh, S.-C.; Yang, J.-H.; Dolgor, E.; Noh, J.-P. High Electrochemical Performance Silicon Thin-Film Free-Standing Electrodes Based on Buckypaper for Flexible Lithium-Ion Batteries. Materials 2021, 14, 2053. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Kanagaraj, A.B.; Al Nahyan, M.S.; Al Shibli, H.; Ashoor, A.A.; Fadaq, H.; Al Dahmani, S.; Choi, D.S. Electrical and electrochemical properties of carbon nanotube-based free standing LTO electrodes for current collector-free Li-ion batteries. Curr. Appl. Phys. 2019, 19, 1150–1155. [Google Scholar] [CrossRef]







| LC40 | LC50 | LC60 | LTO_Cu | LC40 after | LC50 after | LC60 after | LTO_Cu after | |
|---|---|---|---|---|---|---|---|---|
| Rs (Ω) | 4.81 | 5.2 | 4.64 | 3.52 | 5.91 | 6.18 | 5.82 | 3.42 |
| Rct(Ω) | 90.55 | 98.88 | 107.78 | 169.7 | 21.74 | 35.34 | 62.15 | 130.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Yun, S.-D.; Nyamaa, O.; Yang, J.-H.; Huh, S.-C.; Jeong, H.-M.; Nam, T.-H.; Ryu, Y.-J.; Noh, J.-P. Free-Standing Li4Ti5O12/Carbon Nanotube Electrodes for Flexible Lithium-Ion Batteries. Energies 2022, 15, 8585. https://doi.org/10.3390/en15228585
Lee J-S, Yun S-D, Nyamaa O, Yang J-H, Huh S-C, Jeong H-M, Nam T-H, Ryu Y-J, Noh J-P. Free-Standing Li4Ti5O12/Carbon Nanotube Electrodes for Flexible Lithium-Ion Batteries. Energies. 2022; 15(22):8585. https://doi.org/10.3390/en15228585
Chicago/Turabian StyleLee, Jun-Seok, Sang-Du Yun, Oyunbayar Nyamaa, Jeong-Hyeon Yang, Sun-Chul Huh, Hyo-Min Jeong, Tae-Hyun Nam, Yeon-Ju Ryu, and Jung-Pil Noh. 2022. "Free-Standing Li4Ti5O12/Carbon Nanotube Electrodes for Flexible Lithium-Ion Batteries" Energies 15, no. 22: 8585. https://doi.org/10.3390/en15228585
APA StyleLee, J. -S., Yun, S. -D., Nyamaa, O., Yang, J. -H., Huh, S. -C., Jeong, H. -M., Nam, T. -H., Ryu, Y. -J., & Noh, J. -P. (2022). Free-Standing Li4Ti5O12/Carbon Nanotube Electrodes for Flexible Lithium-Ion Batteries. Energies, 15(22), 8585. https://doi.org/10.3390/en15228585