Dairy Wastewater as a Potential Feedstock for Valuable Production with Concurrent Wastewater Treatment through Microbial Electrochemical Technologies
Abstract
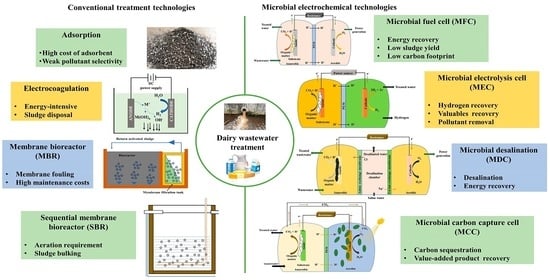
:1. Introduction
2. Characteristics of Dairy Wastewater and Pollutants Present
2.1. Processing Water
2.2. Cleaning Wastewater
2.3. Sanitary Wastewater
3. Environmental Impacts from the Discharge of Dairy Wastewater
4. Processes from Which Wastewater Is Generated with Characteristics
4.1. Operations Involved in Milk Processing
4.1.1. Milk Reception and Storage
4.1.2. Processing of Milk into Dairy Products
4.2. Dairy Effluent Composition
5. Current Treatment Approaches for Dairy Wastewater
5.1. Chemical Treatment
5.2. Physicochemical Processes
5.2.1. Coagulation–Flocculation
5.2.2. Adsorption
5.2.3. Electrocoagulation
5.2.4. Membrane Treatment Technologies
5.3. Biological Treatment
6. Microbial Electrochemical Technologies: Introduction and Working Principle
6.1. Microbial Fuel Cell
6.2. Microbial Electrolysis Cell
6.3. Microbial Desalinization Cell
6.4. Microbial Carbon Capture Cell
7. Dairy Wastewater Treatment Utilizing METs
7.1. MFCs for Bioelectricity Generation using Dairy Effluent
7.2. MECs for Simultaneous H2 Evolution and Dairy Wastewater Treatment
7.3. MDCs for Water Desalination and Dairy Wastewater Treatment
| Type of MET | Wastewater Used | Power Density (W/m2) | Treatment Efficiency | Reference |
|---|---|---|---|---|
| Air-cathode single-chamber MFC | Real dairy wastewater | 0.005 | COD removal of 92.21% | [1] |
| Catalyst-less and mediator-less membrane MFC | Dairy wastewater | 0.062 | COD removal of 90.46% BOD5 removal of 81.72% | [117] |
| Membrane-less MEC | Combined leachate and dairy wastewater | 800 | COD removal of 73% | [121] |
| Single-chamber MEC | Dairy wastewater | 1520 | COD removal of 95% | [120] |
| Three-chamber MDC | Dairy effluent | 0.0020 | Salt removal rate of 0.341 g/L.day | [124] |
7.4. Valuables Recovered through MES using Dairy Wastewater
8. Strategies to Improve the Performance of METs
8.1. Electrode Modifications
8.2. Membrane Modifications
8.3. Different Configurations Employed
8.4. MET-Based Integrated Systems
9. Water Reuse and Circular Economy via METs in the Dairy Industry and Real-Life Applications
9.1. Circular Economy in METs in the Dairy Industry
9.2. Circular Economy in the Dairy Industry via Other Technologies
9.3. Real-life Applications of METs for Dairy Wastewater Treatment
10. Environmental Impact Assessment and Techno-Economic Assessment of METs
11. Challenges Involved and Future Prospects
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhury, P.; Ray, R.N.; Bandyopadhyay, T.K.; Basak, B.; Muthuraj, M.; Bhunia, B. Process Engineering for Stable Power Recovery from Dairy Wastewater Using Microbial Fuel Cell. Int. J. Hydrogen Energy 2021, 46, 3171–3182. [Google Scholar] [CrossRef]
- Burke, N.; Zacharski, K.A.; Southern, M.; Hogan, P.; Ryan, M.P.; Adley, C.C. The dairy industry: Process, monitoring, standards, and quality. In Descriptive Food Science; Díaz, A.V., García-Gimeno, R.M., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef] [Green Version]
- Yonar, T.; Sivrioğlu, Ö.; Özengin, N. Physico-Chemical Treatment of Dairy Industry Wastewaters: A Review. In Technological Approaches for Novel Applications in Dairy Processing; Koca, N., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef] [Green Version]
- Dongre, A.; Sogani, M.; Sonu, K.; Syed, Z.; Sharma, G. Treatment of Dairy Wastewaters: Evaluating Microbial Fuel Cell Tools and Mechanism. In Environmental Issues and Sustainable Development; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Choudhury, P.; Narayan Ray, R.; Nath Tiwari, O.; Kanti Bandyopadhyay, T.; Muthuraj, M.; Bhunia, B. Strategies for Improvement of Microbial Fuel Cell Performance via Stable Power Generation from Real Dairy Wastewater. Fuel 2021, 288, 119653. [Google Scholar] [CrossRef]
- Choudhury, P.; Ray, R.N.; Bandyopadhyay, T.K.; Tiwari, O.N.; Bhunia, B. Kinetics and Performance Evaluation of Microbial Fuel Cell Supplied with Dairy Wastewater with Simultaneous Power Generation. Int. J. Hydrogen Energy 2021, 46, 16815–16822. [Google Scholar] [CrossRef]
- Sanjay, S.; Udayashankara, T.H. Dairy Wastewater Treatment with Bio-Electricity Generation Using Dual Chambered Membrane-Less Microbial Fuel Cell. Mater. Today Proc. 2021, 35, 308–311. [Google Scholar] [CrossRef]
- Selvasembian, R.; Mal, J.; Rani, R.; Sinha, R.; Agrahari, R.; Joshua, I.; Santhiagu, A.; Pradhan, N. Recent Progress in Microbial Fuel Cells for Industrial Effluent Treatment and Energy Generation: Fundamentals to Scale-up Application and Challenges. Bioresour. Technol. 2022, 346, 126462. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, I.S.; Shah, M.P. Bioremediation approaches for treatment of pulp and paper industry wastewater: Recent advances and challenges. In Microbial Bioremediation & Biodegradation; Shah, M.P., Ed.; Springer: Singapore, 2020; pp. 1–48. [Google Scholar] [CrossRef]
- Rikame, S.S.; Mungray, A.A.; Mungray, A.K. Electricity Generation from Acidogenic Food Waste Leachate Using Dual Chamber Mediator Less Microbial Fuel Cell. Int. Biodeterior. Biodegrad. 2012, 75, 131–137. [Google Scholar] [CrossRef]
- Sogani, M.; Pankan, A.O.; Dongre, A.; Yunus, K.; Fisher, A.C. Augmenting the Biodegradation of Recalcitrant Ethinylestradiol Using Rhodopseudomonas palustris in a Hybrid Photo-Assisted Microbial Fuel Cell with Enhanced Bio-Hydrogen Production. J. Hazard Mater. 2021, 408, 124421. [Google Scholar] [CrossRef]
- Slavov, A.K. General Characteristics and Treatment Possibilities of Dairy Wastewater—A Review. Food Technol. Biotechnol. 2017, 55, 14. [Google Scholar] [CrossRef]
- Kaur, N. Different Treatment Techniques of Dairy Wastewater. Groundw. Sustain. Dev. 2021, 14, 100640. [Google Scholar] [CrossRef]
- Akansha, J.; Nidheesh, P.V.; Gopinath, A.; Anupama, K.V.; Suresh Kumar, M. Treatment of Dairy Industry Wastewater by Combined Aerated Electrocoagulation and Phytoremediation Process. Chemosphere 2020, 253, 126652. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Cohen, B. The Bacterial Culture as an Electrical Half-Cell. Available online: https://scholar.google.com/scholar_lookup?author=B.+Cohen+&publication_year=1931&title=The+bacterial+culture+as+an+electrical+half-cell&journal=J.+Bacteriol&volume=21&pages=18-19 (accessed on 28 September 2022).
- Davis, J.B. Generation of Electricity by Microbial Action. Adv. Appl. Microbiol. 1963, 5, 51–64. [Google Scholar] [PubMed]
- Davis, J.B.; Yarbrough, H.F. Preliminary Experiments on a Microbial Fuel Cell. Science 1962, 137, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, F.; Schroder, U. From MFC to MXC: Chemical and Biological Cathodes and Their Potential for Microbial Bioelectrochemical Systems. Chem. Soc. Rev. 2010, 39, 4433–4448. [Google Scholar] [CrossRef]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Dominguez, X.; Strik, D.P.B.T.B.; Sarma, P.M. An Overview on Emerging Bioelectrochemical Systems (BESs): Technology for Sustainable Electricity, Waste Remediation, Resource Recovery, Chemical Production and Beyond. Renew Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Das, I.; Ghangrekar, M.M. Application of Bioelectrochemical Systems for Carbon Dioxide Sequestration and Concomitant Valuable Recovery: A Review. Mater. Sci. Energy Technol. 2019, 2, 687–696. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Ahmad, A.; Das, S.; Madhao Ghangrekar, M. Application of Microbial Electrochemical Technologies for the Treatment of Petrochemical Wastewater with Concomitant Valuable Recovery: A Review. Environ. Sci. Pollut. Res. 2021, 29, 61783–61802. [Google Scholar] [CrossRef]
- Hernandez, C.A.; Osma, J.F. Microbial Electrochemical Systems: Deriving Future Trends from Historical Perspectives and Characterization Strategies. Front. Environ. Sci. 2020, 8, 44. [Google Scholar] [CrossRef]
- Schröder, U.; Harnisch, F.; Angenent, L.T. Microbial Electrochemistry and Technology: Terminology and Classification. Energy Environ. Sci. 2015, 8, 513–519. [Google Scholar] [CrossRef]
- Pant, D.; van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A Review of the Substrates Used in Microbial Fuel Cells (MFCs) for Sustainable Energy Production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Nancharaiah, Y.v.; Venkata Mohan, S.; Lens, P.N.L. Metals Removal and Recovery in Bioelectrochemical Systems: A Review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, J.; Kennes, C.; Cheng, Z.; Ye, J.; Chen, D.; Chen, J.; Wang, L. Current Advances of VOCs Degradation by Bioelectrochemical Systems: A Review. Chem. Eng. J. 2018, 334, 2625–2637. [Google Scholar] [CrossRef]
- Sevda, S.; Sreekishnan, T.R.; Pous, N.; Puig, S.; Pant, D. Bioelectroremediation of Perchlorate and Nitrate Contaminated Water: A Review. Bioresour. Technol. 2018, 255, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Arredondo, M.; Kuntke, P.; Jeremiasse, A.W.; Sleutels, T.H.J.A.; Buisman, C.J.N.; ter Heijne, A. Bioelectrochemical Systems for Nitrogen Removal and Recovery from Wastewater. Environ. Sci. 2015, 1, 22–33. [Google Scholar] [CrossRef]
- Janet Joshiba, G.; Senthil Kumar, P.; Femina, C.C.; Jayashree, E.; Racchana, R.; Sivanesan, S. Critical Review on Biological Treatment Strategies of Dairy Wastewater. Desalination Water Treat. 2019, 160, 94–109. [Google Scholar] [CrossRef]
- Shete, B.; Shinkar, N. Dairy Industry Wastewater Sources, Characteristics & Its Effects on Environment. Int. J. Curr. Eng. Technol. 2013, 3, 1611–1615, ISSN 2277-4106. [Google Scholar]
- Tikariha, A.; Sahu, O. Study of Characteristics and Treatments of Dairy Industry Waste Water. J. Appl. Environ. Microbiol. 2014, 2, 16–22. [Google Scholar] [CrossRef]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An Overview of Various Technologies for the Treatment of Dairy Wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef]
- Adesra, A.; Srivastava, V.K.; Varjani, S. Valorization of Dairy Wastes: Integrative Approaches for Value Added Products. Indian J. Microbiol. 2021, 61, 270–278. [Google Scholar] [CrossRef]
- Kolhe, A.S.; Ingale, S.R.; Bhole, R.V. Effluent of Dairy Technology. Int. Res. J. Sodh Samiksha Mulyankan 2008, 4, 303–306. [Google Scholar]
- Onet, C. Characteristics of the Untreated Wastewater Produced by Food Industry. An. Univ. Din Oradea Fasc. Protecţia Mediu. 2010, XV, 709–714. [Google Scholar]
- Das, P.; Paul, K.K. Present Scenario of Dairy Wastewater Treatment: A State of Art Review. 2022, 1–22. Available online: https://www.researchsquare.com/article/rs-1774888/v1 (accessed on 28 September 2022). [CrossRef]
- Jeanson, S.; Floury, J.; Gagnaire, V.; Lortal, S.; Thierry, A. Bacterial Colonies in Solid Media and Foods: A Review on Their Growth and Interactions with the Micro-Environment. Front. Microbiol. 2015, 6, 1284. [Google Scholar] [CrossRef] [Green Version]
- Milani, F.X.; Nutter, D.; Thoma, G. Invited Review: Environmental Impacts of Dairy Processing and Products: A Review. J. Dairy Sci. 2011, 94, 4243–4254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Haynes, R.J. Origin, Nature, and Treatment of Effluents from Dairy and Meat Processing Factories and the Effects of Their Irrigation on the Quality of Agricultural Soils. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1531–1599, ISBN 1064338100. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [Green Version]
- Britz, T.J.; van Schalkwyk, C.; Hung, Y.T. Treatment of Dairy Processing Wastewaters. In Handbook of Industrial and Hazardous Wastes Treatment; CRC Press: Boca Raton, FL, USA, 2004; pp. 673–705. [Google Scholar] [CrossRef]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A Review on Anaerobic Biofilm Reactors for the Treatment of Dairy Industry Wastewater. Process Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef]
- Janczukowicz, W.; Zieliński, M.; Debowski, M. Biodegradability Evaluation of Dairy Effluents Originated in Selected Sections of Dairy Production. Bioresour. Technol. 2008, 99, 4199–4205. [Google Scholar] [CrossRef]
- Rosenwinkel, K.H.; Austermann-Haun, U.; Meyer, H. Industrial Wastewater Sources and Treatment Strategies; Wiley Online Library: New York, NY, USA, 2005. [Google Scholar]
- Doble, M.; Kumar, A. Treatment of Waste from Food and Dairy Industries. Biotreatment of Industrial Effluents; Elsevier: Burlington, MA, USA, 2005; pp. 183–187. [Google Scholar] [CrossRef]
- Finnegan, W.; Clifford, E.; Goggins, J.; O’Leary, N.; Dobson, A.; Rowan, N.; Xiao, L.; Miao, S.; Fitzhenry, K.; Leonard, P.; et al. DairyWater: Striving for Sustainability within the Dairy Processing Industry in the Republic of Ireland. J. Dairy Res. 2018, 85, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Enayaty-Ahangar, F.; Murphy, S.I.; Martin, N.H.; Wiedmann, M.; Ivanek, R. Optimizing Pasteurized Fluid Milk Shelf-Life Through Microbial Spoilage Reduction. Front. Sustain. Food Syst. 2021, 5, 140. [Google Scholar] [CrossRef]
- Watkins, M.; Nash, D. Dairy Factory Wastewaters, Their Use on Land and Possible Environmental Impacts—A Mini Review. Open Agric. J. 2014, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, P.; Kale, V.; Sarkar, B.; Chakrabarti, P.P.; Vijaykumar, A. Wastewater Treatment in Dairy Industries—Possibility of Reuse. Desalination 2006, 195, 141–152. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese Whey Management: A Review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Pesta, G.; Meyer-Pittroff, R.; Russ, W. Utilization of Whey. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: New York, NY, USA, 2007; pp. 193–207. [Google Scholar] [CrossRef]
- Goff, H.D.; Davidson, V.J.; Cappi, E. Viscosity of Ice Cream Mix at Pasteurization Temperatures. J. Dairy Sci. 1994, 77, 2207–2213. [Google Scholar] [CrossRef]
- Jouki, M.; Jafari, S.; Jouki, A.; Khazaei, N. Characterization of Functional Sweetened Condensed Milk Formulated with Flavoring and Sugar Substitute. Food Sci. Nutr. 2021, 9, 5119. [Google Scholar] [CrossRef]
- El-Shamy, S.; Farag, M.A. Volatiles Profiling in Heated Cheese as Analyzed Using Headspace Solid-Phase Microextraction Coupled to Gas Chromatography Coupled to Mass Spectrometry. eFood 2022, 3, e2. [Google Scholar] [CrossRef]
- D’Souza, N.M.; Mawson, A.J. Membrane Cleaning in the Dairy Industry: A Review. Crit. Rev. Food Sci. Nutr. 2005, 45, 125–134. [Google Scholar] [CrossRef]
- Schifrin, S.M.; Ivanov, G.V.; Mishukov, B.G.; Feodanov, Y.A. Wastewaters from dairy industry. In Wastewater Treatment of Meat and Dairy Industry; Light and Food Industry: Moscow, Russia, 1981. [Google Scholar]
- Danalewich, J.R.; Papagiannis, T.G.; Belyea, R.L.; Tumbleson, M.E.; Raskin, L. Characterization of Dairy Waste Streams, Current Treatment Practices, and Potential for Biological Nutrient Removal. Water Res. 1998, 32, 3555–3568. [Google Scholar] [CrossRef]
- Jördening, H.J.; Winter, J. Environmental biotechnology: Concepts and applications. In Environmental Biotechnology: Concepts and Applications; Jördening, H.-J., Winter, J., Eds.; Wiley: Meinheim, Germany, 2005; pp. 1–463. [Google Scholar] [CrossRef]
- Wang, S.; Chandrasekhara Rao, N.; Qiu, R.; Moletta, R. Performance and Kinetic Evaluation of Anaerobic Moving Bed Biofilm Reactor for Treating Milk Permeate from Dairy Industry. Bioresour. Technol. 2009, 100, 5641–5647. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, R.; McGarvey, J.A.; Benemann, J.R. Biohydrogen Production from Cheese Processing Wastewater by Anaerobic Fermentation Using Mixed Microbial Communities. Int. J. Hydrogen Energy 2007, 32, 4761–4771. [Google Scholar] [CrossRef]
- Karama, B. Solid and Liquid Waste Management from Dairy Industry. Master’s Thesis, University of Science and Technology, Omdurman, Sudan, 2018. [Google Scholar]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic Treatment of Dairy Wastewaters: A Review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Shubham, E. Characterization And Treatment of Ice Cream Industry Wastewater Using UASB Reactor. Int. J. New Technol. Sci. Eng. 2015, 2, 69–77. [Google Scholar]
- Shammas, N.K.; Wang, L.K.; Hahn, H.H. Fundamentals of Wastewater Flotation. In Flotation Technology; Humana Press: Totowa, NJ, USA, 2010; pp. 121–164. [Google Scholar] [CrossRef]
- Nadais, M.H.G.A.G.; Capela, M.I.A.P.F.; Arroja, L.M.G.A.; Hung, Y.-T. Anaerobic Treatment of Milk Processing Wastewater; Springer: Totowa, NJ, USA, 2010; pp. 555–627. [Google Scholar] [CrossRef]
- Muruganandam, L.; Kumar, S.; Jena, A.; Gulla, S.; Godhwani, B. Treatment of Waste Water by Coagulation and Flocculation Using Biomaterials. IOP Conf. Series: Mater. Sci. Eng. 2017, 263, 032006. [Google Scholar] [CrossRef]
- Selmer-Olsen, E.; Ratnaweera, H.C.; Pehrson, R. A Novel Treatment Process for Dairy Wastewater with Chitosan Produced Shrimp-Shell Waste. Water Sci. Technol. 1996, 34, 33–40. [Google Scholar] [CrossRef]
- Rao, M.; Bhole, A.G. Removal of Organic Matter from Dairy Industry Waste Water Using Low-Cost Adsorbents. Indian Chem. Eng. 2002, 44, 25–28. [Google Scholar]
- Pathak, U.; Das, P.; Banerjee, P.; Datta, S. Treatment of Wastewater from a Dairy Industry Using Rice Husk as Adsorbent: Treatment Efficiency, Isotherm, Thermodynamics, and Kinetics Modelling. J. Thermodyn. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Şengil, I.A.; Özacar, M. Treatment of Dairy Wastewaters by Electrocoagulation Using Mild Steel Electrodes. J. Hazard Mater. 2006, 137, 1197–1205. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Moein, H.; Kord Mostafapour, F.; Nakhaie, S. Application of Electrocoagulation Process for Dairy Wa stewater Treatment. J. Chem. 2013, 2013, 640139. [Google Scholar] [CrossRef] [Green Version]
- Frappart, M.; Akoum, O.; Ding, L.H.; Jaffrin, M.Y. Treatment of Dairy Process Waters Modelled by Diluted Milk Using Dynamic Nanofiltration with a Rotating Disk Module. J. Memb. Sci. 2006, 282, 465–472. [Google Scholar] [CrossRef]
- Vourch, M.; Balannec, B.; Chaufer, B.; Dorange, G. Treatment of Dairy Industry Wastewater by Reverse Osmosis for Water Reuse. Desalination 2008, 219, 190–202. [Google Scholar] [CrossRef]
- Kumari, R.; Ankit, H.; Basu, S. Reclamation of Water from Dairy Wastewater Using Membrane Bioreactor (MBR)—Membrane Filtration Processes. Mater Today Proc. 2021, 47, 1452–1456. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, Y.T.; Lo, H.H.; Yapijakis, C. Waste Treatment in the Food Processing Industry; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Banu, J.R.; Kaliappan, S.; Yeom, I.T. Two-Stage Anaerobic Treatment of Dairy Wastewater Using HUASB with PUF and PVC Carrier. Biotechnol. Bioprocess Eng. 2007, 12, 257–264. [Google Scholar] [CrossRef]
- Carta-Escobar, F.; Pereda-Marín, J.; Álvarez-Mateos, P.; Romero-Guzmán, F.; Durán-Barrantes, M.M.; Barriga-Mateos, F. Aerobic Purification of Dairy Wastewater in Continuous Regime: Part I: Analysis of the Biodegradation Process in Two Reactor Configurations. Biochem. Eng. J. 2004, 21, 183–191. [Google Scholar] [CrossRef]
- Pescod, M.B. Wastewater Treatment and Use in Agriculture-FAO Irrigation and Drainage Paper 47; Food and Agriculture Organization of the United Nations: Rome, Italy, 1922. [Google Scholar]
- Neczaj, E.; Kacprzak, M.; Kamizela, T.; Lach, J.; Okoniewska, E. Sequencing Batch Reactor System for the Co-Treatment of Landfill Leachate and Dairy Wastewater. Desalination 2008, 222, 404–409. [Google Scholar] [CrossRef]
- Perle, M.; Kimchie, S.; Shelef, G. Some Biochemical Aspects of the Anaerobic Degradation of Dairy Wastewater. Water Res. 1995, 29, 1549–1554. [Google Scholar] [CrossRef]
- Dugba, P.N.; Zhang, R. Treatment of Dairy Wastewater with Two-Stage Anaerobic Sequencing Batch Reactor Systems—Thermophilic versus Mesophilic Operations. Bioresour. Technol. 1999, 68, 225–233. [Google Scholar] [CrossRef]
- Gavala, H.N.; Kopsinis, H.; Skiadas, I.v.; Stamatelatou, K.; Lyberatos, G. Treatment of Dairy Wastewater Using an Upflow Anaerobic Sludge Blanket Reactor. J. Agric. Eng. Res. 1999, 73, 59–63. [Google Scholar] [CrossRef]
- Borja, R.; Banks, C.J. Kinetics of an Upflow Anaerobic Sludge Blanket Reactor Treating Ice-Cream Wastewater. Environ. Technol. 1994, 15, 219–232. [Google Scholar] [CrossRef]
- Nadais, H.; Capela, I.; Arroja, L.; Duarte, A. Treatment of Dairy Wastewater in UASB Reactors Inoculated with Flocculent Biomass. Water SA 2005, 31, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Loloei, M.; Alidadi, H.; Nekonam, G.; Kor, Y. Study of the Coagulation Process in Wastewater Treatment of Dairy Industries. Int. J. Environ. Health Eng. 2014, 3, 12. [Google Scholar] [CrossRef]
- Al-Ananzeh, N.M. Treatment of Wastewater from a Dairy Plant by Adsorption Using Synthesized Copper Oxide Nanoparticles: Kinetics and Isotherms Modeling Optimization. Water Sci. Technol. 2021, 83, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, R. Aerobic Treatment of Dairy Wastewater with Sequencing Batch Reactor Systems. Bioprocess Biosyst. Eng. 2002, 25, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.M.; Ahmad, K.S. Bioelectrochemical Systems: Sustainable Bio-Energy Powerhouses. Biosens. Bioelectron. 2019, 142, 111576. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A State of the Art Review on Microbial Fuel Cells: A Promising Technology for Wastewater Treatment and Bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Kiely, P.D.; Regan, J.M.; Logan, B.E. The Electric Picnic: Synergistic Requirements for Exoelectrogenic Microbial Communities. Curr. Opin. Biotechnol. 2011, 22, 378–385. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2013, 339, 906. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, M.; Zhou, M.; Yang, H.; Liang, L.; Gu, T. Microbial Fuel Cell Hybrid Systems for Wastewater Treatment and Bioenergy Production: Synergistic Effects, Mechanisms and Challenges. Renew. Sustain. Energy Rev. 2019, 103, 13–29. [Google Scholar] [CrossRef]
- Kasipandian, K.; Saigeetha, S.; Samrot, A.V.; Abirami, S.; Emilin Renitta, R.; Dhiva, S. Bioelectricity Production Using Microbial Fuel Cell—A Review. Biointerface Res. Appl. Chem. 2021, 11, 9420–9431. [Google Scholar] [CrossRef]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Su Lim, S.; Ismail, M. Non-Pt Catalyst as Oxygen Reduction Reaction in Microbial Fuel Cells: A Review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Al-saned, A.J.O.; Kitafa, B.A.; Badday, A.S. Microbial Fuel Cells (MFC) in the Treatment of Dairy Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 012073. [Google Scholar] [CrossRef]
- Azuma, M.; Ojima, Y. Catalyst Development of Microbial Fuel Cells for Renewable-Energy Production. In Current Topics in Biochemical Engineering; Shiomi, N., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Erable, B.; Féron, D.; Bergel, A. Microbial Catalysis of the Oxygen Reduction Reaction for Microbial Fuel Cells: A Review. ChemSusChem 2012, 5, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent Advances and Emerging Challenges in Microbial Electrolysis Cells (MECs) for Microbial Production of Hydrogen and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Microbial Electrolysis Cells Turning to Be Versatile Technology: Recent Advances and Future Challenges. Water Res. 2014, 56, 11–25. [Google Scholar] [CrossRef]
- Kadier, A.; Jain, P.; Lai, B.; Kalil, M.S.; Kondaveeti, S.; Alabbosh, K.F.S.; Abu-Reesh, I.M.; Mohanakrishna, G. Biorefinery Perspectives of Microbial Electrolysis Cells (MECs) for Hydrogen and Valuable Chemicals Production through Wastewater Treatment. Biofuel Res. J. 2020, 7, 1128–1142. [Google Scholar] [CrossRef] [Green Version]
- Saeed, H.M.; Husseini, G.A.; Yousef, S.; Saif, J.; Al-Asheh, S.; Abu Fara, A.; Azzam, S.; Khawaga, R.; Aidan, A. Microbial Desalination Cell Technology: A Review and a Case Study. Desalination 2015, 359, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A Comprehensive Review of Microbial Electrochemical Systems as a Platform Technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Kim, Y.; Logan, B.E. Microbial Desalination Cells for Energy Production and Desalination. Desalination 2013, 308, 122–130. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Ahmad, W.; Baawain, M.S.; Khadem, M.; Dhar, B.R. A Review of Microbial Desalination Cell Technology: Configurations, Optimization and Applications. J. Clean. Prod. 2018, 183, 458–480. [Google Scholar] [CrossRef]
- Luo, H.; Xu, P.; Roane, T.M.; Jenkins, P.E.; Ren, Z. Microbial Desalination Cells for Improved Performance in Wastewater Treatment, Electricity Production, and Desalination. Bioresour. Technol. 2012, 105, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Liu, J.; Lee, H.; Li, C.; Li, N.; Ren, N. Sequestration of CO2 Discharged from Anode by Algal Cathode in Microbial Carbon Capture Cells (MCCs). Biosens. Bioelectron. 2010, 25, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Ling, T.C.; Juan, J.C.; Lee, D.J.; Chang, J.S.; Show, P.L. Biorefineries of Carbon Dioxide: From Carbon Capture and Storage (CCS) to Bioenergies Production. Bioresour. Technol. 2016, 215, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Nayak, B.K.; Das, D. Microbial Carbon Capture Cell Using Cyanobacteria for Simultaneous Power Generation, Carbon Dioxide Sequestration and Wastewater Treatment. Bioresour. Technol. 2012, 107, 97–102. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Liu, B. Effect of Algal Species and Light Intensity on the Performance of an Air-Lift-Type Microbial Carbon Capture Cell with an Algae-Assisted Cathode. RSC Adv. 2016, 6, 25094–25100. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Jain, S.C.; Ghangrekar, M.M. Simultaneous Wastewater Treatment, Algal Biomass Production and Electricity Generation in Clayware Microbial Carbon Capture Cells. Appl. Biochem. Biotechnol. 2017, 183, 1076–1092. [Google Scholar] [CrossRef]
- Ahmad, A.; Das, S.; Ghangrekar, M.M. Removal of Xenobiotics from Wastewater by Electrocoagulation: A Mini-Review. J. Indian Chem. Soc. 2019, 97, 493–500. [Google Scholar]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J.; Khanjani, N. Evaluation of Dairy Industry Wastewater Treatment and Simultaneous Bioelectricity Generation in a Catalyst-Less and Mediator-Less Membrane Microbial Fuel Cell. J. Saudi Chem. Soc. 2016, 20, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Marassi, R.J.; Queiroz, L.G.; Silva, D.C.V.R.; dos Santos, F.S.; Silva, G.C.; de Paiva, T.C.B. Long-Term Performance and Acute Toxicity Assessment of Scaled-up Air–Cathode Microbial Fuel Cell Fed by Dairy Wastewater. Bioprocess Biosyst. Eng. 2020, 43, 1561–1571. [Google Scholar] [CrossRef]
- Das, S.; Ghangrekar, M.M. Tungsten Oxide as Electrocatalyst for Improved Power Generation and Wastewater Treatment in Microbial Fuel Cell. Environ. Technol. 2019, 41, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Rani, G.; Banu, J.R.; Kumar, G.; Yogalakshmi, K.N. Statistical Optimization of Operating Parameters of Microbial Electrolysis Cell Treating Dairy Industry Wastewater Using Quadratic Model to Enhance Energy Generation. Int. J. Hydrogen Energy 2022, 47, 37401–37414. [Google Scholar] [CrossRef]
- Rani, G.; Nabi, Z.; Rajesh Banu, J.; Yogalakshmi, K.N. Batch Fed Single Chambered Microbial Electrolysis Cell for the Treatment of Landfill Leachate. Renew Energy 2020, 153, 168–174. [Google Scholar] [CrossRef]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Anusha, G.; Noori, M.T.; Ghangrekar, M.M. Application of Silver-Tin Dioxide Composite Cathode Catalyst for Enhancing Performance of Microbial Desalination Cell. Mater. Sci. Energy Technol. 2018, 1, 188–195. [Google Scholar] [CrossRef]
- Zamanpour, M.K.; Kariminia, H.R.; Vosoughi, M. Electricity Generation, Desalination and Microalgae Cultivation in a Biocathode-Microbial Desalination Cell. J. Environ. Chem. Eng. 2017, 5, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Das, I.; Ghangrekar, M.M. Role of Applied Potential on Microbial Electrosynthesis of Organic Compounds through Carbon Dioxide Sequestration. J. Environ. Chem. Eng. 2020, 8, 104028. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, P.; Ghangrekar, M.M. Increasing Methane Content in Biogas and Simultaneous Value Added Product Recovery Using Microbial Electrosynthesis. Water Sci. Technol. 2018, 77, 1293–1302. [Google Scholar] [CrossRef]
- Georgiou, S.; Koutsokeras, L.; Constantinou, M.; Majzer, R.; Markiewicz, J.; Siedlecki, M.; Vyrides, I.; Constantinides, G. Microbial Electrosynthesis Inoculated with Anaerobic Granular Sludge and Carbon Cloth Electrodes Functionalized with Copper Nanoparticles for Conversion of CO2 to CH4. Nanomaterials 2022, 12, 2472. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent Advances in the Use of Different Substrates in Microbial Fuel Cells toward Wastewater Treatment and Simultaneous Energy Recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity Generation from Artificial Wastewater Using an Upflow Microbial Fuel Cell. ACS Publ. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial Fuel Cells: Novel Biotechnology for Energy Generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Li, B. The Variation of Power Generation with Organic Substrates in Single-Chamber Microbial Fuel Cells (SCMFCs). Bioresour. Technol. 2010, 101, 1844–1850. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Yakhmi, J.V. Microbial Fuel Cells–Applications for Generation of Electrical Power and Beyond. Crit. Rev. Microbiol. 2016, 42, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. ACS Publications 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Zhou, X.; Wang, X.; Li, B.; Santoro, C.; Grattieri, M.; Babanova, S.; Artyushkova, K.; Atanassov, P.; et al. Surface Modification of Microbial Fuel Cells Anodes: Approaches to Practical Design. Electrochim. Acta 2014, 134, 116–126. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Mishra, B.K. The Effects of Microbial Fuel Cell Integration into Constructed Wetland on the Performance of Constructed Wetland. Bioresour. Technol. 2015, 195, 223–230. [Google Scholar] [CrossRef]
- Vidhyeswari, D.; Bhuvaneshwari, S. Desalination and Water Treatment Treatment of Dairy Wastewater and Performance Comparison of Three Different Electrodes in Microbial Fuel Cell System. Desalination Water Treat. 2018, 122, 15–16. [Google Scholar] [CrossRef] [Green Version]
- Sekar, A.D.; Jayabalan, T.; Muthukumar, H.; Chandrasekaran, N.I.; Mohamed, S.N.; Matheswaran, M. Enhancing Power Generation and Treatment of Dairy Waste Water in Microbial Fuel Cell Using Cu-Doped Iron Oxide Nanoparticles Decorated Anode. Energy 2019, 172, 173–180. [Google Scholar] [CrossRef]
- Mahdi Mardanpour, M.; Nasr Esfahany, M.; Behzad, T.; Sedaqatvand, R. Single Chamber Microbial Fuel Cell with Spiral Anode for Dairy Wastewater Treatment. Biosens. Bioelectron. 2012, 38, 264–269. [Google Scholar] [CrossRef]
- Noori, M.T.; Ghangrekar, M.M.; Mukherjee, C.K. V2O5 Microflower Decorated Cathode for Enhancing Power Generation in Air-Cathode Microbial Fuel Cell Treating Fish Market Wastewater. Int. J. Hydrog. Energy 2016, 41, 3638–3645. [Google Scholar] [CrossRef]
- An, J.; Jeon, H.; Lee, J.; Chang, I.S. Bifunctional Silver Nanoparticle Cathode in Microbial Fuel Cells for Microbial Growth Inhibition with Comparable Oxygen Reduction Reaction Activity. ACS Publ. 2011, 45, 5441–5446. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shi, X.; Wang, X.; Lee, H.; Liu, J.; Qu, Y.; He, W.; Kumar, S.S.; Kim, B.H.; Ren, N. Effects of Sulfide on Microbial Fuel Cells with Platinum and Nitrogen-Doped Carbon Powder Cathodes. Biosens. Bioelectron. 2012, 35, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.; Rabaey, K.; Keller, J.; Buisman, C.J. Towards Practical Implementation of Bioelectrochemical Wastewater Treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Veeramani, V.; Rajangam, K.; Nagendran, J. Performance of Cobalt Oxide/Carbon Cloth Composite Electrode in Energy Generation from Dairy Wastewater Using Microbial Fuel Cells. Sustain. Environ. Res. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Gao, F.; Yang, L.; Kehoe, D.K.; Romeral, L.; Gun’ko, Y.K.; Lyons, M.G.; Wang, J.J.; Mullarkey, D.; et al. Characterising and Control of Ammonia Emission in Microbial Fuel Cells. Chem. Eng. J. 2020, 389, 124462. [Google Scholar] [CrossRef]
- Li, W.W.; Sheng, G.P.; Liu, X.W.; Yu, H.Q. Recent Advances in the Separators for Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion Exchange Membranes as Separators in Microbial Fuel Cells for Bioenergy Conversion: A Comprehensive Review. Renew. Sustain. Energy Rev. 2011, 28, 575–587. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; De Los Ríos, A.P.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Lozano-Blanco, L.J.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent Progress and Perspectives in Microbial Fuel Cells for Bioenergy Generation and Wastewater Treatment. Fuel Processing Technology 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Asensio, Y.; Fernandez-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Influence of the Ion-Exchange Membrane on the Performance of Double-Compartment Microbial Fuel Cells. J. Electroanal. Chem. 2018, 808, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Ayyaru, S.; Dharmalingam, S. Development of MFC Using Sulphonated Polyether Ether Ketone (SPEEK) Membrane for Electricity Generation from Waste Water. Bioresour. Technol. 2011, 102, 11167–11171. [Google Scholar] [CrossRef] [PubMed]
- Vidhyeswari, D.; Surendhar, A.; Bhuvaneshwari, S. Evaluation of Power Generation and Treatment Efficiency of Dairy Wastewater in Microbial Fuel Cell Using TiO2—SPEEK as Proton Exchange Membrane. Water Sci. Technol. 2021, 84, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Lóránt, B.; Gyalai-Korpos, M.; Goryanin, I.; Tardy, G.M. Application of Air Cathode Microbial Fuel Cells for Energy Efficient Treatment of Dairy Wastewater. Period. Polytech. Chem. Eng. 2021, 65, 200–209. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Influence of Reactor’s Hydrodynamics on the Performance of Microbial Fuel Cells. J. Water Process Eng. 2018, 26, 281–288. [Google Scholar] [CrossRef]
- Marassi, R.J.; Queiroz, L.G.; Silva, D.C.V.R.; da Silva, F.T.; Silva, G.C.; de Paiva, T.C.B. Performance and Toxicity Assessment of an Up-Flow Tubular Microbial Fuel Cell during Long-Term Operation with High-Strength Dairy Wastewater. J. Clean. Prod. 2020, 259, 120882. [Google Scholar] [CrossRef]
- Wenzel, J.; Fuentes, L.; Cabezas, A.; Etchebehere, C. Microbial Fuel Cell Coupled to Biohydrogen Reactor: A Feasible Technology to Increase Energy Yield from Cheese Whey. Bioprocess Biosyst. Eng. 2017, 40, 807–819. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, C.H.; Wang, C.T.; Wang, Y.T. Effect of FeSO4 on Bio-Electro-Fenton Microbial Fuel Cells with Different Exchange Membranes. Mater. Res. Innov. 2015, 19, S51276–S51279. [Google Scholar] [CrossRef]
- Bolognesi, S.; Cecconet, D.; Callegari, A.; Capodaglio, A.G. Combined Microalgal Photobioreactor/Microbial Fuel Cell System: Performance Analysis under Different Process Conditions. Environ. Res. 2021, 192, 110263. [Google Scholar] [CrossRef]
- Visvanathan, C. Hazardous Waste Disposal. Resour. Conserv. Recycl. 1996, 16, 201–212. [Google Scholar] [CrossRef]
- Mohan, S.V.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A Circular Bioeconomy with Biobased Products from CO2 Sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.N.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Sarpong, G.; Gude, V.G. Transitioning Wastewater Treatment Plants toward Circular Economy and Energy Sustainability. ACS Omega 2021, 6, 11794–11803. [Google Scholar] [CrossRef]
- Lehtoranta, S.; Laukka, V.; Vidal, B.; Heiderscheidt, E.; Postila, H.; Nilivaara, R.; Herrmann, I. Circular Economy in Wastewater Management—The Potential of Source-Separating Sanitation in Rural and Peri-Urban Areas of Northern Finland and Sweden. Front. Environ. Sci. 2022, 10, 97. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Lloyd, J.R.; Scott, K.; Premier, G.C.; Eileen, H.Y.; Curtis, T.; Head, I.M. A Critical Review of Integration Analysis of Microbial Electrosynthesis (MES) Systems with Waste Biorefineries for the Production of Biofuel and Chemical from Reuse of CO2. Renew. Sustain. Energy Rev. 2016, 56, 116–132. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar]
- Muddasar, M.; Liaquat, R.; Abdullah, A.; Khoja, A.H.; Shahzad, N.; Iqbal, N.; Ali, M.I.; Uddin, A.; Ullah, S. Evaluating the Use of Unassimilated Bio-Anode with Different Exposed Surface Areas for Bioenergy Production Using Solar-Powered Microbial Electrolysis Cell. Int. J. Energy Res. 2021, 45, 20143–20155. [Google Scholar] [CrossRef]
- Mehrotra, S.; Kiran Kumar, V.; Man mohan, K.; Gajalakshmi, S.; Pathak, B. Bioelectrogenesis from Ceramic Membrane-Based Algal-Microbial Fuel Cells Treating Dairy Industry Wastewater. Sustain. Energy Technol. Assess. 2021, 48, 101653. [Google Scholar] [CrossRef]
- Theodoridis, G.; Pechlivanis, A.; Thomaidis, N.S.; Spyros, A.; Georgiou, C.A.; Albanis, T.; Skoufos, I.; Kalogiannis, S.; Tsangaris, G.T.; Stasinakis, A.S. FoodOmicsGR_RI: A Consortium for Comprehensive Molecular Characterisation of Food Products. Metabolites 2021, 11, 74. [Google Scholar] [CrossRef]
- Vasyliv, O.M.; Bilyy, O.I.; Ferensovych, Y.P.; Hnatush, S.O. Application of Acetate, Lactate, and Fumarate as Electron Donors in Microbial Fuel Cell. SPIE 2013, 8825, 170–176. [Google Scholar] [CrossRef]
- Teng, S.-X.; Tong, Z.-H.; Li, W.-W.; Wang, S.-G.; Sheng, G.-P.; Shi, X.-Y.; Liu, W.; Yu, H.-Q. Electricity Generation from Mixed Volatile Fatty Acids Using Microbial Fuel Cells. Appl. Microbiol. Biotechnol. 2010, 87, 2365–2372. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. Assessing the Feasibility of N and P Recovery by Struvite Precipitation from Nutrient-Rich Wastewater: A Review. Environ. Sci. Pollut. Res. 2015, 22, 17453–17464. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A.; Heckman, J.R.; Lew, B.; Rouff, A.A. Magnesium Supplementation for Improved Struvite Recovery from Dairy Lagoon Wastewater. J. Environ. Chem. Eng. 2021, 9, 105628. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The Biostimulation of Anaerobic Digestion with (Semi)Conductive Ferric Oxides: Their Potential for Enhanced Biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Chen, P.; Yu, J.; Liu, J.; Ye, J.; Zhou, S. Recyclable Magnetite-Enhanced Electromethanogenesis for Biomethane Production from Wastewater. Water Res. 2019, 166, 115095. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Microalgae-Biorefinery with Cascading Resource Recovery Design Associated to Dairy Wastewater Treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Zielí Nski, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Swica, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Callegari, A.; Cecconet, D.; Molognoni, D.; Capodaglio, A.G. Sustainable Processing of Dairy Wastewater: Long-Term Pilot Application of a Bio-Electrochemical System. J. Clean. Prod. 2018, 189, 563–569. [Google Scholar] [CrossRef]
- Drisya, C.M.; Manjunath, N.T. Dairy Wastewater Treatment and Electricity Generation Using Microbial Fuel Cell. Int. Res. J. Eng. Technol. 2017, 4, 1293–1296. [Google Scholar]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of Techno-Economic Analysis and Life Cycle Assessment for Sustainable Process Design—A Review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; van Bogaert, G.; Gallego, Y.A.; Diels, L.; Vanbroekhoven, K. An Introduction to the Life Cycle Assessment (LCA) of Bioelectrochemical Systems (BES) for Sustainable Energy and Product Generation: Relevance and Key Aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- Savla, N.; Suman; Pandit, S.; Verma, J.P.; Awasthi, A.K.; Sana, S.S.; Prasad, R. Techno-Economical Evaluation and Life Cycle Assessment of Microbial Electrochemical Systems: A Review. Curr. Res. Green Sustain. Chem. 2021, 4, 100111. [Google Scholar] [CrossRef]
- Yu, J.; Park, Y.; Widyaningsih, E.; Kim, S.; Kim, Y.; Lee, T. Microbial Fuel Cells: Devices for Real Wastewater Treatment, Rather than Electricity Production. Sci. Total Environ. 2021, 775, 145904. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Deng, Y.; Zha, Y.; Abu-Reesh, I.M.; He, Z.; Yuan, C. Life Cycle Assessment of a Microbial Desalination Cell for Sustainable Wastewater Treatment and Saline Water Desalination. J. Clean. Prod. 2018, 200, 900–910. [Google Scholar] [CrossRef]
- Chin, M.Y.; Phuang, Z.X.; Woon, K.S.; Hanafiah, M.M.; Zhang, Z.; Liu, X. Life Cycle Assessment of Bioelectrochemical and Integrated Microbial Fuel Cell Systems for Sustainable Wastewater Treatment and Resource Recovery. J. Environ. Manag. 2022, 320, 115778. [Google Scholar] [CrossRef]
- Sharma, M.; Bajracharya, S.; Gildemyn, S.; Patil, S.A.; Alvarez-Gallego, Y.; Pant, D.; Rabaey, K.; Dominguez-Benetton, X. A Critical Revisit of the Key Parameters Used to Describe Microbial Electrochemical Systems. Electrochim. Acta 2014, 140, 191–208. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Arkatkar, A.; Kumar, S.S. Recent Advancement in Scaling-up Applications of Microbial Fuel Cells: From Reality to Practicability. Sustain. Energy Technol. Assess. 2021, 45, 101226. [Google Scholar] [CrossRef]
- Shah, M.; Petrovski, S.; Coutor, R. Emerging and Innovative Technologies for Water and Wastewater Treatment. Lett. Appl. Microbiol. 2022, 75, 700. [Google Scholar] [CrossRef]
- Syed, Z.; Sogani, M.; Dongre, A.; Kumar, A.; Sonu, K.; Sharma, G.; Gupta, A.B. Bioelectrochemical Systems for Environmental Remediation of Estrogens: A Review and Way Forward. Sci. Total Environ. 2021, 780, 146544. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Tsimas, E.S.; Barampouti, E.M.P.; Mai, S.T. Anaerobic Digestion of Cheese Dairy Wastewater Following Chemical Oxidation. Biosyst. Eng. 2012, 113, 253–258. [Google Scholar] [CrossRef]




| Review Article Reference | Type of Pollutant Covered | Prevailing Technologies for Pollutant Treatment | Application of METs for Pollutant Removal | Electrode Modifications | Membrane Modifications | Different Configuration for Pollutant Treatment | Integrated System Approach | Circular Economy in METs | Environmental Impact Assessment of METs |
|---|---|---|---|---|---|---|---|---|---|
| [26] | Heavy metals | No | Yes | No | No | No | Yes | No | No |
| [27] | Volatile organic compounds (VOCs) | No | Yes | No | No | Yes | No | No | No |
| [28] | Perchlorate and nitrate | No | Yes | No | No | No | No | No | No |
| [29] | Nitrogen removal | No | Yes | No | No | No | No | No | No |
| [22] | Petrochemical wastewater | Yes | Yes | No | No | No | Yes | No | No |
| Current review | Dairy wastewater | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Stages in the Processing of Milk | Sources of Wastewater | pH | BOD5 (g/L) | COD (g/L) | TSS (g/L) | TN (g/L) | TP (g/L) | References |
|---|---|---|---|---|---|---|---|---|
| Milk receiving stage | a. Poor drainage facility b. Cleaning of units c. Spillage and overflows d. Foaming | 7.18 | 0.8–5 | 2.54–10 | 0.65–3 | - | - | [43,45] |
| Butter production process | a. Vacreation process (pasteurisation by vacuum methods) b. Use of salts increases salinity and ions such as Na+ and Cl− c. Cleaning and washing operations | 12.08 | 0.22–2.65 | 8.93–10.2 | 0.7–5.07 | - | - | [45,63] |
| Cheese making | a. Whey separation b. Cleaning and washing operations c. Usage of salts tends to increase ionic concentration and suspended solids d. Spillages and leaks | 3.38-9.5 | 0.59–5 | 1–63.3 | 0.19–2.5 | 0.018–0.83 | 0.005–0.28 | [45,50,64] |
| Ice cream production process | a. Plant and tank clean-up b. Backflushing water c. Pasteurizer and chiller flush-out | 5.1–6.96 | 1.8–2.45 | 4.94–5.2 | 1.1–3.1 | 0.014–0.06 | - | [43,62,65] |
| Treatment Technology | Process Conditions | Treatment Efficiency | Drawbacks | Reference |
|---|---|---|---|---|
| Coagulation and flocculation | Polyacrylamide and polyferric sulfate as coagulants, pH = 7.5, coagulant dose = 20 mg/L | 95% turbidity removal 82% COD removal |
| [88] |
| Adsorption | Synthesized copper oxide nanoparticles coupled with Sophora Japonica fruit as adsorbent, contact time = 120 min, temperature = 25 °C, pH = 7.5, adsorbent dose = 1 g/L | 77 to 95% COD removal |
| [89] |
| Electrocoagulation | Six aluminium electrodes in parallel connection, voltage input = 60 V, maximum current = 5 A, HRT = 60 min | 98.84% COD removal 97.95% BOD5 removal 97.75% TSS removal |
| [74] |
| Reverse osmosis | RO membrane area = 540 m2, transmembrane pressure = 20 bar | 95% Water recovery 99.8% TOC removal |
| [76] |
| Membrane bioreactor | MBR with PVDF membrane of 0.2 to 0.3 μm pore size, water flux = 4 to 7 L/h, HRT = 6 h, organic loading = 20 to 22 g/L, pH = 6.5–7 | 99.8% COD removal 98% BOD5 removal 40% TDS removal 80% NH4-N removal 98.7% PO4 removal |
| [77] |
| Sequencing batch reactor | Initial COD = 20,000 mg/L, HRT = 2 days | 80.2% COD removal 63.4% TS removal 66.2% VS removal 75% TKN removal 38.3% TN removal |
| [90] |
| Upflow anaerobic sludge blanket reactor | Organic loading rate = 6.2 g COD/L.day, reactor volume = 10 L, HRT = 6 day | 98% COD removal |
| [85] |
| Strategy | Modification of Material/System | Type of MET | Power Density | Removal Efficiency | Reference |
|---|---|---|---|---|---|
| Electrode modification | Anode decorated with copper-doped iron oxide nanoparticles | Dual-compartment MFC | 161.5 mW/m2 | COD removal of 75% | [137] |
| Copper-blended 3D cathode | Air-cathode MFC | 14.4 W/m3 | COD removal of 88.1% | [144] | |
| Membranes | Sulfonated polyether ether ketone | Single-chamber MFC | 5.7 W/m3 | COD removal of 75% | [149] |
| TiO2-SPEEK membrane | Dual-chamber MFC | 1.22 W/m2 | COD removal of 90% | [150] | |
| Configurations | - | Air-cathode-single chamber MFC | 170 mW/m3 | COD removal of 71.1% | [151] |
| - | Dual-chamber MFC | 12.21 W/m3 | COD removal of 80.9% | [152] | |
| - | Conventional-three chamber MDC | 20.25 mW/m2 | Salt removal rate of 0.341 g/L.day | [124] | |
| Integrated systems | Integration with dark fermentation | Single-chamber MFC | 439 mW/m2 | COD removal of 42% | [154] |
| Integration with electro-Fenton process | Dual-chamber MFC | 260 mW/m2 | COD removal of 77% | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganta, A.; Bashir, Y.; Das, S. Dairy Wastewater as a Potential Feedstock for Valuable Production with Concurrent Wastewater Treatment through Microbial Electrochemical Technologies. Energies 2022, 15, 9084. https://doi.org/10.3390/en15239084
Ganta A, Bashir Y, Das S. Dairy Wastewater as a Potential Feedstock for Valuable Production with Concurrent Wastewater Treatment through Microbial Electrochemical Technologies. Energies. 2022; 15(23):9084. https://doi.org/10.3390/en15239084
Chicago/Turabian StyleGanta, Anusha, Yasser Bashir, and Sovik Das. 2022. "Dairy Wastewater as a Potential Feedstock for Valuable Production with Concurrent Wastewater Treatment through Microbial Electrochemical Technologies" Energies 15, no. 23: 9084. https://doi.org/10.3390/en15239084
APA StyleGanta, A., Bashir, Y., & Das, S. (2022). Dairy Wastewater as a Potential Feedstock for Valuable Production with Concurrent Wastewater Treatment through Microbial Electrochemical Technologies. Energies, 15(23), 9084. https://doi.org/10.3390/en15239084