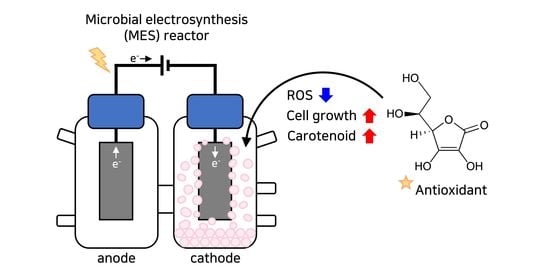
Modulation of Antioxidant Activity Enhances Photoautotrophic Cell Growth of Rhodobacter sphaeroides in Microbial Electrosynthesis †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Preparation of Microbial Electrosynthesis Reactor
2.2. Transcriptome Analysis
2.3. Determination of ROS Levels
2.4. Measurement of Total Carotenoid Contents
2.5. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Growth Behavior According to Reducing Source in R. sphaeroides
3.2. Enhancement of Bacterial Cell Growth and Carotenoid Production by Modulating ROS in MES
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Oh, Y.K.; Lee, S.; Fitriana, H.N.; Moon, M.; Kim, M.S.; Lee, J.; Min, K.; Park, G.W.; Lee, J.P.; et al. Recent developments and key barriers to microbial CO2 electrobiorefinery. Bioresour. Technol. 2021, 320, 124350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, Y.; Song, T.; Xie, J. Bioplastic production from the microbial electrosynthesis of acetate through CO2 reduction. Energy Fuels 2021, 35, 15978–15986. [Google Scholar] [CrossRef]
- Wu, H.; Pan, H.; Li, Z.; Liu, T.; Liu, F.; Xiu, S.; Wang, J.; Wang, H.; Hou, Y.; Yang, B.; et al. Efficient production of lycopene from CO2 via microbial electrosynthesis. Chem. Eng. J. 2022, 430, 132943. [Google Scholar] [CrossRef]
- Krieg, T.; Sydow, A.; Faust, S.; Huth, I.; Holtmann, D. CO2 to Terpenes: Autotrophic and electroautotrophic α-humulene production with Cupriavidus necator. Angew. Chem. Int. Ed. 2018, 57, 1879–1882. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Balaguer, M.D.; Colprim, J. Continuous acetate production through microbial electrosynthesis from CO2 with microbial mixed culture. J. Chem. Technol. Biotechnol. 2016, 91, 921–927. [Google Scholar] [CrossRef]
- Schmid, F.; Novion Ducassou, J.; Couté, Y.; Gescher, J. Developing Rhodobacter sphaeroides for cathodic biopolymer production. Bioresour. Technol. 2021, 336, 125340. [Google Scholar] [CrossRef]
- Orsi, E.; Folch, P.L.; Monje-López, V.T.; Fernhout, B.M.; Turcato, A.; Kengen, S.W.M.; Eggink, G.; Weusthuis, R.A. Characterization of heterotrophic growth and sesquiterpene production by Rhodobacter sphaeroides on a defined medium. J. Ind. Microbiol. Biotechnol. 2019, 46, 1179–1190. [Google Scholar] [CrossRef] [Green Version]
- Su, A.; Chi, S.; Li, Y.; Tan, S.; Qiang, S.; Chen, Z.; Meng, Y. Metabolic redesign of Rhodobacter sphaeroides for lycopene production. J. Agric. Food Chem. 2018, 66, 5879–5885. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.P.; Fleury, M.J.J. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef]
- Kars, G.; Demirel Kars, M.; Obali, İ.; Emsen, A.; Gündüz, U. Investigation of antioxidant and cytotoxic effects of biotechnologically produced carotenoids from Rhodobacter sphaeroides O.U. 001. Gümüşhane Üniversitesi Fen Bilimleri Dergisi 2020, 10, 559–568. [Google Scholar] [CrossRef]
- Tian, B.; Xu, Z.; Sun, Z.; Lin, J.; Hua, Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Farsaraei, S.; Moghaddam, M. Application of exogenous ascorbic acid modifies growth and pigment content of Calendula officinalis L. flower heads of plants exposed to NaCl Stress. J. Soil Sci. Plant Nutr. 2021, 21, 2803–2814. [Google Scholar] [CrossRef]
- Sistrom, W.R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J. Gen. Microbiol. 1962, 28, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Fitriana, H.N.; Lee, J.; Lee, S.; Moon, M.; Lee, Y.R.; Oh, Y.K.; Park, M.; Lee, J.S.; Song, J.; Lee, S.Y. Surface modification of a graphite felt cathode with amide-coupling enhances the electron uptake of Rhodobacter sphaeroides. Appl. Sci. 2021, 11, 7585. [Google Scholar] [CrossRef]
- Lee, Y.R.; Nur Fitriana, H.; Lee, S.Y.; Kim, M.S.; Moon, M.; Lee, W.H.; Lee, J.S.; Lee, S. Molecular profiling and optimization studies for growth and PHB production conditions in Rhodobacter sphaeroides. Energies 2020, 13, 6471. [Google Scholar] [CrossRef]
- Li, S.; Sakuntala, M.; Song, Y.E.; Heo, J.O.; Kim, M.; Lee, S.Y.; Kim, M.S.; Oh, Y.K.; Kim, J.R. Photoautotrophic hydrogen production of Rhodobacter sphaeroides in a microbial electrosynthesis cell. Bioresour. Technol. 2021, 320, 124333. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Liu, J.; Wang, Y.; Bi, C.; Zhang, X. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate from glucose and CO2. Microb. Cell Fact. 2019, 18, 15. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.; Ma, C.; Yang, Z.; Li, Z.; Yang, X.; Wang, M.; Zhang, H.; Hu, J.; Zhang, C. Photosynthetic bacteria-based technology is a potential alternative to meet sustainable wastewater treatment requirement? Environ. Int. 2020, 137, 105417. [Google Scholar] [CrossRef]
- Assil-Companioni, L.; Büchsenschütz, H.C.; Solymosi, D.; Dyczmons-Nowaczyk, N.G.; Bauer, K.K.F.; Wallner, S.; MacHeroux, P.; Allahverdiyeva, Y.; Nowaczyk, M.M.; Kourist, R. Engineering of NADPH Supply Boosts Photosynthesis-Driven Biotransformations. ACS Catal. 2020, 10, 11864–11877. [Google Scholar] [CrossRef]
- Nikkanen, L.; Solymosi, D.; Jokel, M.; Allahverdiyeva, Y. Regulatory electron transport pathways of photosynthesis in cyanobacteria and microalgae: Recent advances and biotechnological prospects. Physiol. Plant. 2021, 173, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Agledal, L.; Niere, M.; Ziegler, M. The phosphate makes a difference: Cellular functions of NADP. Redox Rep. 2010, 15, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, C.S.; Armiger, W.B.; Dodds, D.R.; Dordick, J.S.; Koffas, M.A.G. Improved strategies for electrochemical 1,4-NAD(P)H2 regeneration: A new era of bioreactors for industrial biocatalysis. Biotechnol. Adv. 2018, 36, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W.M. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6, 742. [Google Scholar] [CrossRef]
- Ghirardi, M.L.; Posewitz, M.C.; Maness, P.C.; Dubini, A.; Yu, J.; Seibert, M. Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annu. Rev. Plant Biol. 2007, 58, 71–91. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, Y.S.; Shin, W.R.; Yu, J.; Lee, J.; Lee, S.; Kim, Y.H.; Min, J. Non-photosynthetic CO2 bio-mitigation by: Escherichia coli harbouring CBB genes. Green Chem. 2020, 22, 6889–6896. [Google Scholar] [CrossRef]
- Chou, M.E.; Chang, W.T.; Chang, Y.C.; Yang, M.K. Expression of four pha genes involved in poly-β-hydroxybutyrate production and accumulation in Rhodobacter sphaeroides FJ1. Mol. Genet. Genom. 2009, 282, 97–106. [Google Scholar] [CrossRef]
- Baker, A.E.; O’Toole, G.A. Bacteria, rev your engines: Stator dynamics regulate flagellar motility. J. Bacteriol. 2017, 199, e00088-17. [Google Scholar] [CrossRef] [Green Version]
- del Pilar Anzola Rojas, M.; Mateos, R.; Sotres, A.; Zaiat, M.; Gonzalez, E.R.; Escapa, A.; De Wever, H.; Pant, D. Microbial electrosynthesis (MES) from CO2 is resilient to fluctuations in renewable energy supply. Energy Convers. Manag. 2018, 177, 272–279. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef]
- Ziegelhoffer, E.C.; Donohue, T.J. Bacterial responses to photo-oxidative stress. Nat. Rev. Microbiol. 2009, 7, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torella, J.P.; Gagliardi, C.J.; Chen, J.S.; Bediako, D.K.; Colón, B.; Way, J.C.; Silver, P.A.; Nocera, D.G. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system Proc. Natl. Acad. Sci. USA 2015, 112, E1507. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 2016, 352, 602–692. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Opgenorth, P.H.; Wernick, D.G.; Rogers, S.; Wu, T.Y.; Higashide, W.; Malati, P.; Huo, Y.X.; Cho, K.M.; Liao, J.C. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 2012, 335, 1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Li, F.; Tian, Y.; Song, H. Enzyme-assisted microbial electrosynthesis of poly(3-hydroxybutyrate) via CO2 bioreduction by engineered Ralstonia eutropha. ACS Catal. 2018, 8, 4429–4437. [Google Scholar] [CrossRef]
- Gallie, D.R. L-Ascorbic acid: A multifunctional molecule supporting plant growth and development. Sientifica 2013, 2013, 795964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Harbinson, J. Relationships between antioxidant metabolism and carotenoids in the regulation of photosynthesis. In The Photochemistry of Carotenoids; Springer: Dordrecht, The Netherlands, 2006; pp. 305–325. [Google Scholar] [CrossRef]
- Kim, H.; Tong, X.; Choi, S.; Lee, J.K. Characterization of ATPase activity of free and immobilized chromatophore membrane vesicles of Rhodobacter sphaeroides. J. Microbiol. Biotechnol. 2017, 27, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- Uchoa, A.F.; Knox, P.P.; Turchielle, R.; Seifullina, N.K.; Baptista, M.S. Singlet oxygen generation in the reaction centers of Rhodobacter sphaeroides. Eur. Biophys. J. 2008, 37, 843–850. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Zhao, Q.; Ge, C.; Dai, S.; Wang, Q.H. Diversity of nitrate, oxalate, vitamin C and carotenoid contents in different spinach accessions and their correlation with various morphological traits. J. Hortic. Sci. Biotechnol. 2018, 93, 409–415. [Google Scholar] [CrossRef]




| Gene Number | Gene Name | Function | Description | Log2(FC) |
|---|---|---|---|---|
| RSP_0495 | hupS | Hydrogenase protein small subunit | Energy production and conversion | −7.6 |
| RSP_0496 | hupL | Hydrogenase protein large subunit | Energy production and conversion | −9.7 |
| RSP_1281 | cbbS | Ribulose 1,5-bisphosphate carboxylase small subunit | Carbohydrate transport and metabolism | −6.0 |
| RSP_1282 | cbbL | Ribulose 1,5-bisphosphate carboxylase large subunit | Energy production and conversion | −6.3 |
| RSP_1283 | cfxA | Fructose-1,6-bisphosphate aldolase | Carbohydrate transport and metabolism | −6.5 |
| RSP_1284 | prkA | Phosphoribulokinase | Energy production and conversion | −6.7 |
| RSP_1285 | fbp1 | Fructose-1,6-bisphosphatase | Carbohydrate transport and metabolism | −6.4 |
| RSP_1286 | cbbR | RuBisCO operon transcriptional regulator, CbbR | Transcription | −2.2 |
| RSP_0382 | phaC1 | Poly-beta-hydroxybutyrate polymerase | Lipid transport and metabolism | −2.6 |
| RSP_0745 | phaA | Acetyl-CoA acetyltransferase | Lipid transport and metabolism | −1.8 |
| RSP_0747 | phaB | 3-oxoacyl-(Acyl-carrier-protein) reductase | Function unknown | −3.7 |
| RSP_1257 | phaC2 | Putative polyhydroxyalkanoic synthase | Lipid transport and metabolism | −3.6 |
| RSP_0380 | Polyhydroxyalkanoate synthesis repressor, PhaR | Function unknown | 2.9 | |
| RSP_0034 | flhA | Flagellar biosynthesis protein | Cell motility | 8.4 |
| RSP_0036 | flgA | Flagella basal body P-ring formation protein | Cell motility | 5.9 |
| RSP_0052 | fliE | Flagellar hook-basal body complex protein | Cell motility | 7.0 |
| RSP_0053 | fliF1 | Flagellar M-ring protein | Cell motility | 8.2 |
| RSP_0058 | fliK | FliK, flagellar hook-length control protein | Cell motility | 6.4 |
| RSP_0063 | fliP | Flagellar biosynthetic protein | Cell motility | 9.2 |
| RSP_0065 | fliR | Flagellar biosynthetic protein | Cell motility | 5.8 |
| RSP_0066 | flhB | Flagellar biosynthetic protein | Cell motility | 7.3 |
| RSP_0070 | fliD | Flagellar hook-associated protein 2 | Cell motility | 4.0 |
| RSP_0073 | flgL | Flagellar hook-associated protein 3 | Cell motility | 5.9 |
| RSP_0074 | flgK1 | Flagellar hook-associated protein 1 | Cell motility | 6.7 |
| RSP_0076 | flgI1 | Flagellar P-ring protein | Cell motility | 8.5 |
| RSP_0077 | flgH | Flagellar L-ring protein | Cell motility | 6.0 |
| RSP_0080 | flgE | Flagellar hook protein | Cell motility | 6.6 |
| RSP_0231 | motB | Flagellar MotB protein | Cell motility | 5.9 |
| RSP_0233 | motA | Flagellar MotA protein | Cell motility | 7.7 |
| RSP_2380 | katC | Catalase | Inorganic ion transport and metabolism | −1.3 |
| RSP_2780 | oxyR2 | Transcriptional regulator, LysR family | Transcription | 2.4 |
| RSP_1796 | sodC | Superoxide dismutase [Cu-Zn] | Inorganic ion transport and metabolism | 1.1 |
| RSP_1092 | rpoE | ECF RNA polymerase sigma factor RpoE | Transcription | 2.8 |
| RSP_1093 | chrR | Anti-sigma-E factor ChrR | Transcription | 2.9 |
| RSP_2143 | phrA | DNA photolyase, Cryptochrome 1 apoprotein (Blue light photoreceptor) | Replication, recombination and repair | 4.0 |
| RSP_1194 | grxC | Glutaredoxin | Posttranslational modification, protein turnover | 1.1 |
| RSP_2953 | Glutaredoxin | Posttranslational modification, protein turnover | 1.6 | |
| RSP_1529 | trxA | Thioredoxin | Posttranslational modification, protein turnover | 2.0 |
| RSP_0725 | Thioredoxin | Posttranslational modification, protein turnover | 2.6 | |
| RSP_0264 | crtF | Demethylspheroidene O-methyltransferase | Function unknown | −4.7 |
| RSP_0265 | crtE | Geranylgeranyl diphosphate synthase | Coenzyme transport and metabolism | −6.2 |
| RSP_0266 | crtD | Hydroxyneurosporene desaturase | Secondary metabolites biosynthesis | −3.9 |
| RSP_0267 | crtC | Acyclic carotenoid 1,2-hydratase | Function unknown | −3.2 |
| RSP_0270 | crtB | Phytoene synthase | Lipid transport and metabolism | −3.2 |
| RSP_0271 | crtI | Phytoene desaturase | Secondary metabolites biosynthesis | −3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.R.; Lee, S.Y.; Lee, J.; Kim, H.S.; Lee, J.-S.; Lee, W.-H.; Lee, S. Modulation of Antioxidant Activity Enhances Photoautotrophic Cell Growth of Rhodobacter sphaeroides in Microbial Electrosynthesis. Energies 2022, 15, 935. https://doi.org/10.3390/en15030935
Lee YR, Lee SY, Lee J, Kim HS, Lee J-S, Lee W-H, Lee S. Modulation of Antioxidant Activity Enhances Photoautotrophic Cell Growth of Rhodobacter sphaeroides in Microbial Electrosynthesis. Energies. 2022; 15(3):935. https://doi.org/10.3390/en15030935
Chicago/Turabian StyleLee, Yu Rim, Soo Youn Lee, Jiye Lee, Hui Su Kim, Jin-Suk Lee, Won-Heong Lee, and Sangmin Lee. 2022. "Modulation of Antioxidant Activity Enhances Photoautotrophic Cell Growth of Rhodobacter sphaeroides in Microbial Electrosynthesis" Energies 15, no. 3: 935. https://doi.org/10.3390/en15030935
APA StyleLee, Y. R., Lee, S. Y., Lee, J., Kim, H. S., Lee, J. -S., Lee, W. -H., & Lee, S. (2022). Modulation of Antioxidant Activity Enhances Photoautotrophic Cell Growth of Rhodobacter sphaeroides in Microbial Electrosynthesis. Energies, 15(3), 935. https://doi.org/10.3390/en15030935