Performance of Cs-Doped Carbon-Based Perovskite Solar Cells in Ambient Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Electron Transport Layer Synthesis
2.4. Preparation of the Absorber Layer Precursor Solution
2.5. Preparation of the Carbon Counter Electrode
2.6. Solar Cell Fabrication
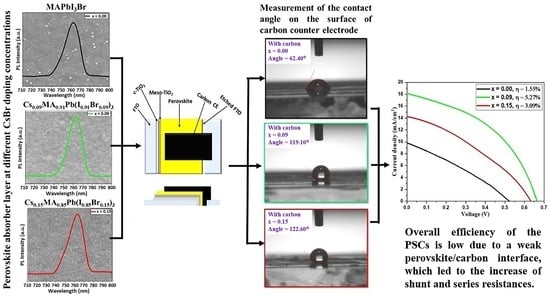
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, H.M.; Reda, S.M.; Ali, A.I.; Mousa, M.A. A quick peek at solar cells and a closer insight at perovskite solar cells. Egypt. J. Pet. 2021, 30, 53–63. [Google Scholar] [CrossRef]
- Qiang, Y.; Xie, Y.; Qi, Y.; Wei, P.; Shi, H.; Geng, C.; Liu, H. Enhanced performance of carbon-based perovskite solar cells with a Li+-doped SnO2 electron transport layer and Al2O3 scaffold layer. Sol. Energy 2020, 201, 523–529. [Google Scholar] [CrossRef]
- Jin, J.; Yang, M.; Deng, W.; Xin, J.; Tai, Q.; Qian, J.; Dong, B.; Li, W.; Wang, J.; Li, J. Highly efficient and stable carbon-based perovskite solar cells with the polymer hole transport layer. Sol. Energy 2021, 220, 491–497. [Google Scholar] [CrossRef]
- Keremane, K.S.; Prathapani, S.; Haur, L.J.; Bahulayan, D.; Adhikari, A.V.; Priyadarshi, A.; Mhaisalkar, S.G. Solvent selection for highly reproducible carbon-based mixed-cation hybrid lead halide perovskite solar cells via adduct approach. Sol. Energy 2020, 199, 761–771. [Google Scholar] [CrossRef]
- Maram, D.K.; Habibiyan, H.; Ghafoorifard, H.; Shekoofa, O. Analysis of Optimum Copper Oxide Hole Transporting Layer for Perovskite Solar Cells. In Proceedings of the 27th Iranian Conference on Electrical Engineering (ICEE), Yazd, Iran, 30 April–2 May 2019. [Google Scholar]
- Zhao, X.-C.; Wu, D.-X.; Yang, L.-J.; Tang, J.; Yue, G.-Z.; Yang, P. Cesium-containing methylammonium lead iodide light absorber for planar perovskite solar cells. J. Nanosci. Nanotechnol. 2020, 20, 1008–1012. [Google Scholar] [CrossRef]
- Maram, D.K.; Haghighi, M.; Shekoofa, O.; Habibiyan, H.; Ghafoorifard, H. A modeling study on utilizing ultra-thin inorganic HTLs in inverted p–n homojunction perovskite solar cells. Sol. Energy 2021, 213, 1–12. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 4 May 2023).
- Han, Q.; Yang, S.; Wang, L.; Yu, F.; Zhang, C.; Wu, M.; Ma, T. The sulfur-rich small molecule boosts the efficiency of carbon-based CsPbI2Br perovskite solar cells to approaching 14%. Sol. Energy 2021, 216, 351–357. [Google Scholar] [CrossRef]
- Nkele, A.C.; Nwanya, A.C.; Shinde, N.M.; Ezugwu, S.; Maaza, M.; Shaikh, J.S.; Ezema, F.I. The use of nickel oxide as a hole transport material in perovskite solar cell configuration: Achieving a high performance and stable device. Int. J. Energy Res. 2020, 44, 9839–9863. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Cheng, L.; Wang, N.; Wang, J.; Gao, F.; Huang, W. High-efficiency flexible solar cells based on organometal halide perovskites. Adv. Mater. 2016, 28, 4532–4540. [Google Scholar] [CrossRef]
- Nkele, A.C.; Ike, I.S.; Ezugwu, S.; Maaza, M.; Ezema, F.I. An overview of the mathematical modelling of perovskite solar cells towards achieving highly efficient perovskite devices. Int. J. Energy Res. 2021, 45, 1496–1516. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic–organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Wei, Y.; Li, W.; Xiang, S.; Liu, J.; Liu, H.; Zhu, L.; Chen, H. Precursor effects on methylamine gas-induced CH3NH3PbI3 films for stable carbon-based perovskite solar cells. Sol. Energy 2018, 174, 139–148. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Wang, B.; Novendra, N.; Navrotsky, A. Energetics, structures, and phase transitions of cubic and orthorhombic cesium lead iodide (CsPbI3) polymorphs. J. Am. Chem. Soc. 2019, 141, 14501–14504. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, J.; Kim, H.-B.; Kim, S.; Walker, B.; Kim, G.-H.; Kim, J.Y. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells. Nano Energy 2014, 7, 80–85. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Wang, X.; Tao, J.; Jiang, J.; Hu, Z.; Chu, J. Enhanced performance of carbon-based planar CsPbBr3 perovskite solar cells with room-temperature sputtered Nb2O5 electron transport layer. Sol. Energy 2019, 191, 263–271. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Jiang, F.; Luo, Q.; Zhang, L.; Tan, M.; Xie, M.; Li, Y.-Q.; Zhou, Y.; Su, W.; et al. Electrochemical corrosion of Ag electrode in the silver grid electrode-based flexible perovskite solar cells and the suppression method. Sol. RRL 2018, 2, 1800118. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef] [Green Version]
- Bidikoudi, M.; Simal, C.; Stathatos, E. Low-toxicity perovskite applications in carbon electrode perovskite solar cells—A review. Electronics 2021, 10, 1145. [Google Scholar] [CrossRef]
- Bogachuk, D.; Zouhair, S.; Wojciechowski, K.; Yang, B.; Babu, V.; Wagner, L.; Xu, B.; Lim, J.; Mastroianni, S.; Pettersson, H.; et al. Low-temperature carbon-based electrodes in perovskite solar cells. Energy Environ. Sci. 2020, 13, 3880–3916. [Google Scholar] [CrossRef]
- Que, M.; Zhang, B.; Chen, J.; Yin, X.; Yun, S. Carbon-based electrodes for perovskite solar cells. Mater. Adv. 2021, 2, 5560–5579. [Google Scholar] [CrossRef]
- Bashir, A.; Haur, J.H.; Shukla, S.; Gupta, D.; Baikie, T.; Chakraborty, S.; Patidar, R.; Bruno, A.; Mhaisalkar, S.; Akhter, Z. Cu-doped nickel oxide interface layer with nanoscale thickness for efficient and highly stable printable carbon-based perovskite solar cell. Sol. Energy 2019, 182, 225–236. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Shi, J.; Su, H.; Luo, Y.; Li, D.; Wu, H.; Cheng, Y.-B.; Meng, Q. Self-adhesive macroporous carbon electrodes for efficient and stable perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1802985. [Google Scholar] [CrossRef]
- Ku, Z.; Rong, Y.; Xu, M.; Liu, T.; Han, H. Full printable processed mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with carbon counter electrode. Sci. Rep. 2013, 3, 3132. [Google Scholar] [CrossRef] [Green Version]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Lyu, B.; Yang, L.; Luo, Y.; Zhang, X.; Zhang, J. Counter electrodes for perovskite solar cells: Materials, interfaces and device stability. J. Mater. Chem. C 2022, 10, 10775–10798. [Google Scholar] [CrossRef]
- Kartikay, P.; Yella, A.; Mallick, S. Binder-solvent effects on low temperature-processed carbon-based, hole-transport layer free perovskite solar cells. Mater. Chem. Phys. 2020, 256, 123594. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.N.; Wall, J.; Li, L.; Xing, G.; Zhang, Q.; Yan, F. Low-temperature processed highly efficient hole transport layer free carbon-based planar perovskite solar cells with SnO2 quantum dot electron transport layer. Mater. Today Phys. 2020, 13, 100204. [Google Scholar] [CrossRef]
- Fan, Y.; Qin, H.; Ye, W.; Liu, M.; Huang, F.; Zhong, D. Improving the stability of methylammonium lead iodide perovskite solar cells by cesium doping. Thin Solid Films 2018, 667, 40–47. [Google Scholar] [CrossRef]
- Murugadoss, G.; Thangamuthu, R.; Vijayaraghavan, S.; Kanda, H.; Ito, S. Caesium—Methyl ammonium mixed-cation lead iodide perovskite crystals: Analysis and application for perovskite solar cells. Electrochim. Acta 2017, 257, 267–280. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhang, Y.; Guan, W.-B.; Wang, K.-F.; Cheng, Z.-X.; Wang, Y.-X. Large enhanced conversion efficiency of perovskite solar cells by CsBr doping. J. Mater. Sci. 2017, 52, 13203–13211. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, K. Charge transport and recombination in perovskite (CH3NH3)PbI3 sensitized TiO2 solar cells. J. Phys. Chem. Lett. 2013, 4, 2880–2884. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the efficiency beyond 20% with a mixed cation perovskite solar cell fabricated in ambient air under controlled humidity. Adv. Energy Mater. 2018, 8, 1700677. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, S.; Buyruk, A.; Eichhorn, J.; Yin, S.; Reus, M.A.; Xiao, T.; Pratap, S.; Liang, S.; Weindl, C.L.; et al. The influence of CsBr on crystal orientation and optoelectronic properties of MAPbI3-based solar cells. ACS Appl. Mater. Interfaces 2022, 14, 2958–2967. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Gordillo, G.; Luis, L.C.; Buitrago, J.P.; Reinoso, M.A. Influence of the Concentration of Cs on the Properties of Thin Films of CsXMA(1-X)PbI3 to be used as Active Layer in Hybrid Solar Cells. In Proceedings of the 7th World Conference on Photovoltaic Energy Conversion (WCPEC), Waikoloa, HI, USA, 10–15 June 2018. [Google Scholar]
- Premkumar, S.; Kundu, K.; Umapathy, S. Impact of cesium in methylammonium lead bromide perovskites: Insights into the microstructures, stability and photophysical properties. Nanoscale 2019, 11, 10292–10305. [Google Scholar] [CrossRef]
- Ng, C.H.; Ripolles, T.S.; Hamada, K.; Teo, S.H.; Lim, H.N.; Bisquert, J.; Hayase, S. Tunable open circuit voltage by engineering inorganic cesium lead bromide/iodide perovskite solar cells. Sci. Rep. 2018, 8, 2482. [Google Scholar] [CrossRef] [Green Version]
- Bahtiar, A.; Yazibarahmah, R.; Aprilia, A.; Hidayat, D. Improved the performance and stability at high humidity of perovskite solar cells by mixed cesium-metylammonium cations. Key Eng. Mater. 2020, 860, 9–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Wang, D.; Zhang, Z.; Qi, X.; Zhu, N.; Liu, G.; Li, X.; Hu, H.; Chen, Z.; et al. High efficiency (16.37%) of cesium bromide—Passivated all-inorganic CsPbI2Br perovskite solar cells. Sol. RRL 2019, 3, 1900254. [Google Scholar] [CrossRef]
- Ledinsky, M.; Schönfeldová, T.; Holovský, J.; Aydin, E.; Hájková, Z.; Landová, L.; Neyková, N.; Fejfar, A.; De Wolf, S. Temperature dependence of the Urbach energy in lead iodide perovskites. J. Phys. Chem. Lett. 2019, 10, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghimire, N.; Bobba, R.S.; Gurung, A.; Reza, K.M.; Laskar, M.A.R.; Lamsal, B.S.; Emshadi, K.; Pathak, R.; Afroz, M.A.; Chowdhury, A.H.; et al. Mitigating open-circuit voltage loss in Pb–Sn low-bandgap perovskite solar cells via additive engineering. ACS Appl. Energy Mater. 2021, 4, 1731–1742. [Google Scholar] [CrossRef]
- Hou, X.; Huang, S.; Ou-Yang, W.; Pan, L.; Sun, Z.; Chen, X. Constructing efficient and stable perovskite solar cells via interconnecting perovskite grains. ACS Appl. Mater. Interfaces 2017, 9, 35200–35208. [Google Scholar] [CrossRef]
- Xing, J.; Wang, Q.; Dong, Q.; Yuan, Y.; Fang, Y.; Huang, J. Ultrafast ion migration in hybrid perovskite polycrystalline thin films under light and suppression in single crystals. Phys. Chem. Chem. Phys. 2016, 18, 30484–30490. [Google Scholar] [CrossRef]
- Yang, B.; Brown, C.C.; Huang, J.; Collins, L.; Sang, X.; Unocic, R.R.; Jesse, S.; Kalinin, S.V.; Belianinov, A.; Jakowski, J.; et al. Enhancing ion migration in grain boundaries of hybrid organic–inorganic perovskites by chlorine. Adv. Funct. Mater. 2017, 27, 1700749. [Google Scholar] [CrossRef]
- Hoque, M.N.F.; He, R.; Warzywoda, J.; Fan, Z. Effects of moisture-based grain boundary passivation on cell performance and ionic migration in organic–inorganic halide perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 30322–30329. [Google Scholar] [CrossRef]
- Singh, R.; Sandhu, S.; Yadav, H.; Lee, J.-J. Stable triple-cation (Cs+–MA+–FA+) perovskite powder formation under ambient conditions for hysteresis-free high-efficiency solar cells. ACS Appl. Mater. Interfaces 2019, 11, 29941–29949. [Google Scholar] [CrossRef]
- Nouri, E.; Mohammadi, M.R.; Lianos, P. Construction of perovskite solar cells using inorganic hole-extracting components. ACS Omega 2018, 3, 46–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Liu, X.; Li, W.; Huang, F.; Peng, Y.; Zhong, J.; Cheng, Y.; Ku, Z. Enhancing the performance and stability of carbon-based perovskite solar cells by the cold isostatic pressing method. RSC Adv. 2017, 7, 48958–48961. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef] [Green Version]
- Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Uddin, A. Perovskite solar cells: Progress and advancements. Energies 2016, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, S. Carbon-based perovskite solar cells without hole transport materials: The front runner to the market? Adv. Mater. 2017, 29, 1603994. [Google Scholar] [CrossRef]
- Wei, H.; Xiao, J.; Yang, Y.; Lv, S.; Shi, J.; Xu, X.; Dong, J.; Luo, Y.; Li, D.; Meng, Q. Free-standing flexible carbon electrode for highly efficient hole-conductor-free perovskite solar cells. Carbon 2015, 93, 861–868. [Google Scholar] [CrossRef]
- Zhu, W.; Bao, C.; Li, F.; Yu, T.; Gao, H.; Yi, Y.; Yang, J.; Fu, G.; Zhou, X.; Zou, Z. A halide exchange engineering for CH3NH3PbI3−xBrx perovskite solar cells with high performance and stability. Nano Energy 2016, 19, 17–26. [Google Scholar] [CrossRef]
- Niemann, R.G.; Gouda, L.; Hu, J.; Tirosh, S.; Gottesman, R.; Cameron, P.J.; Zaban, A. Cs+ incorporation into CH3NH3PbI3 perovskite: Substitution limit and stability enhancement. J. Mater. Chem. A 2016, 4, 17819–17827. [Google Scholar] [CrossRef] [Green Version]
- Atourki, L.; Vega, E.; Marí, B.; Mollar, M.; Ait Ahsaine, H.; Bouabid, K.; Ihlal, A. Role of the chemical substitution on the structural and luminescence properties of the mixed halide perovskite thin MAPbI3−xBrx (0 ≤ x ≤ 1) films. Appl. Surf. Sci. 2016, 371, 112–117. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Li, J.; Liang, X.; Wang, L. Enhancement of thermal stability for perovskite solar cells through cesium doping. RSC Adv. 2017, 7, 17473–17479. [Google Scholar] [CrossRef] [Green Version]








| x | Vmp (V) | Imp (A) | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|---|---|
| 0.00 | 0.306 | 5.07 | 0.530 | 9.56 | 0.29 | 1.55 |
| 0.09 | 0.542 | 11.13 | 0.659 | 18.05 | 0.44 | 5.27 |
| 0.15 | 0.368 | 8.41 | 0.639 | 14.13 | 0.30 | 3.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousaf, T.; Shahzad, N.; Sattar, A.; Tariq, M.A.; Hussain, N.; Khan, Z.S.; Javed, S.; Shahzad, M.I.; Pugliese, D. Performance of Cs-Doped Carbon-Based Perovskite Solar Cells in Ambient Environment. Energies 2023, 16, 4748. https://doi.org/10.3390/en16124748
Yousaf T, Shahzad N, Sattar A, Tariq MA, Hussain N, Khan ZS, Javed S, Shahzad MI, Pugliese D. Performance of Cs-Doped Carbon-Based Perovskite Solar Cells in Ambient Environment. Energies. 2023; 16(12):4748. https://doi.org/10.3390/en16124748
Chicago/Turabian StyleYousaf, Tanzeela, Nadia Shahzad, Abdul Sattar, Muhammad Ali Tariq, Naveed Hussain, Zuhair S. Khan, Sofia Javed, Muhammad Imran Shahzad, and Diego Pugliese. 2023. "Performance of Cs-Doped Carbon-Based Perovskite Solar Cells in Ambient Environment" Energies 16, no. 12: 4748. https://doi.org/10.3390/en16124748
APA StyleYousaf, T., Shahzad, N., Sattar, A., Tariq, M. A., Hussain, N., Khan, Z. S., Javed, S., Shahzad, M. I., & Pugliese, D. (2023). Performance of Cs-Doped Carbon-Based Perovskite Solar Cells in Ambient Environment. Energies, 16(12), 4748. https://doi.org/10.3390/en16124748