Integration of Microalgae-Based Bioenergy Production into a Petrochemical Complex: Techno-Economic Assessment
Abstract
:1. Introduction
2. Microalgal Production Plant Siting
2.1. Proposed Location and Site Description
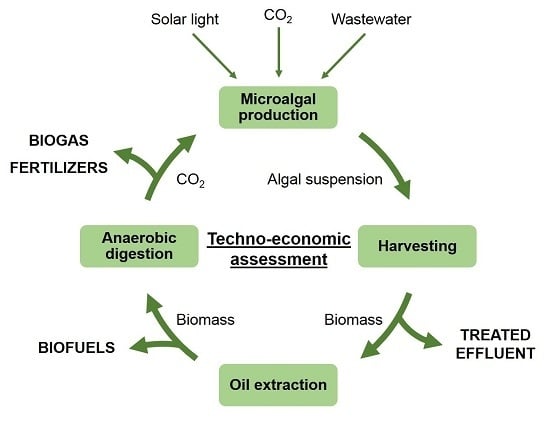
3. Process Flowsheet and Scenarios Description
3.1. Process Flowsheet Description
3.2. Scenarios Description
4. Techno-Economic Assessment
4.1. Mass Balance
4.1.1. Microalgal Growth
4.1.2. Microalgal Harvesting
4.1.3. Cell Disruption and Lipids Extraction
4.1.4. Anaerobic Digestion
4.1.5. Net CO2 Balance
4.2. Energy Balance
4.3. Economic Assessment
4.3.1. Fixed Capital
4.3.2. Annual Production Costs
4.3.3. Annual Revenues
4.3.4. Economic Viability
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trends in Atmospheric Carbon Dioxide. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 19 February 2016).
- Allen, M.R.; Frame, D.J.; Huntingford, C.; Jones, C.D.; Lowe, J.A.; Meinshausen, M.; Meinshausen, N. Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 2009, 458, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Bilanovic, D.; Andargatchew, A.; Kroeger, T.; Shelef, G. Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations—Response surface methodology analysis. Energy Conv. Manag. 2009, 50, 262–267. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The potential for carbon capture. Bioscience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. Green fuel production: Processes applied to microalgae. Environ. Chem. Lett. 2013, 11, 315–324. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Energy requirements and economic analysis of a full-scale microbial flocculation system for microalgal harvesting. Chem. Eng. Res. Des. 2010, 88, 988–996. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Hu, H.; Zhang, X.; Yu, Y.; Chen, Y. Growth and lipid accumulation properties of a freshwater microalga, Chlorella ellipsoidea YJ1, in domestic secondary effluents. Appl. Energy 2011, 88, 3295–3299. [Google Scholar] [CrossRef]
- Lin, L.; Chan, G.; Jiang, B.; Lan, C. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, E.-M.; Phang, S.-M.; Chu, W.-L. Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J. Appl. Phycol. 2012, 24, 953–963. [Google Scholar] [CrossRef]
- González, L.E.; Cañizares, R.O.; Baena, S. Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour. Technol. 1997, 60, 259–262. [Google Scholar] [CrossRef]
- Lefebvre, S.; Hussenot, J.; Brossard, N. Water treatment of land-based fish farm effluents by outdoor culture of marine diatoms. J. Appl. Phycol. 1996, 8, 193–200. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Trace element removal Spirulina sp. from copper smelter and refinery effluents. Hydrometallurgy 2004, 73, 147–153. [Google Scholar] [CrossRef]
- Woertz, I.; Feffer, A.; Lundquist, T.; Nelson, Y. Algae grown on dairy and municipal wastewater for simultaneous nutrient removal and lipid production for biofuel feedstock. J. Environ. Eng. 2009, 135, 1115–1122. [Google Scholar] [CrossRef]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Silva-Benavides, A.; Torzillo, G. Nitrogen and phosphorus removal through laboratory batch cultures of microalga Chlorella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J. Appl. Phycol. 2012, 24, 267–276. [Google Scholar] [CrossRef]
- Benemann, J.R. Hydrogen production by microalgae. J. Appl. Phycol. 2000, 12, 291–300. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.; Banerjee, U. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkouski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007; pp. 5–21. [Google Scholar]
- Pires, J.C.M.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Simões, M. Wastewater treatment to enhance the economic viability of microalgae culture. Environ. Sci. Pollut. Res. 2013, 20, 5096–5105. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.W.; Johnson, M.D.; Zhang, X.; Zemke, P.; Chen, W.; Hu, Q. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Slegers, P.; Leduc, S.; Wijffels, R.; van Straten, G.; van Boxtel, A. Logistic analysis of algae cultivation. Bioresour. Technol. 2015, 179, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Venteris, E.R.; McBride, R.C.; Coleman, A.M.; Skaggs, R.L.; Wigmosta, M.S. Siting algae cultivation facilities for biofuel production in the United States: Trade-offs between growth rate, site constructability, water availability, and infrastructure. Environ. Sci. Technol. 2014, 48, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Densidade Populacional Segundo os Censos em Portugal. Available online: http://www.pordata.pt/Portugal/Densidade+populacional+segundo+os+Censos-412 (accessed on 5 March 2013). (In Portugese)
- Kumar, A.; Ergas, S.; Yuan, X.; Sahu, A.; Zhang, Q.; Dewulf, J.; Malcata, F.X.; van Langenhove, H. Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Photovoltaic Geographical Information system—Incident Global Irradiation. Available online: http://re.jrc.ec.europa.eu/pvgis/apps4/pvest.php (accessed on 20 Jaunary 2013).
- Rodrigues, C.M.M. Cálculo da evaporação de albufeiras de grande regularização do sul de Portugal. Ph.D. Thesis, Universidade de Évora, Évora, Portugal, October 2009. [Google Scholar]
- Chazarra, A.; Barceló, A.M.; Pires, V.; Cunha, S.; Mendes, M.; Neto, J. Iberian Climate Atlas—Air Temperature and Precipitation (1971–2000); AEMET-IM—Agencia Estatal de Meteorología de España and Instituto de Meteorologia de Portugal: Madrid, Spain, 2001. [Google Scholar]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, and fermenters. Prog. Ind. Microbiol. 1999, 35, 313–321. [Google Scholar] [CrossRef]
- Posten, C.; Schaub, G. Microalgae and terrestrial biomass as source for fuels—A process view. J. Biotechnol. 2009, 142, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar] [PubMed]
- Lee, Y.-K. Microalgal mass culture systems and methods: their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Ugwu, C.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Cossı́o, B.R.; Labella, D.; Niell, F.X. The feasibility of industrial production of Spirulina (Arthrospira) in Southern Spain. Aquaculture 2003, 217, 179–190. [Google Scholar] [CrossRef]
- Tredici, M.R.; Materassi, R. From open ponds to vertical alveolar panels: The Italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. J. Appl. Phycol. 1992, 4, 221–231. [Google Scholar] [CrossRef]
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.-P.; Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Melo, A.C.; Simões, M.; Caetano, N.S. Parametric study of a brewery effluent treatment by microalgae Scenedesmus obliquus. Bioresour. Technol. 2012, 107, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Park, S.Y.; Li, Y.B. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sust. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Flisar, K.; Meglic, S.H.; Morelj, J.; Golob, J.; Miklavcic, D. Testing a prototype pulse generator for a continuous flow system and its use for E. coli inactivation and microalgae lipid extraction. Bioelectrochemistry 2014, 100, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Joannes, C.; Sipaut, C.; Dayou, J.; Yasir, S.; Mansa, R. Review Paper on Cell Membrane Electroporation of Microalgae Using Electric Field Treatment Method for Microalgae Lipid Extraction. In Proceedings of the 9th CUTSE International Conference, Miri, Sarawak, Malaysia, 3–4 December 2014; IOP Publishing: Miri, Sarawak, Malaysia, 2015. [Google Scholar]
- Show, K.-Y.; Lee, D.-J.; Tay, J.-H.; Lee, T.-M.; Chang, J.-S. Microalgal drying and cell disruption—Recent advances. Bioresour. Technol. 2015, 184, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, V.; Craggs, R.; Rintala, J. Influence of temperature and pretreatments on the anaerobic digestion of wastewater grown microalgae in a laboratory-scale accumulating-volume reactor. Water Res. 2014, 57, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.C.; Mendoza Martin, J.L.; Heaven, S.; Banks, C.J.; Acien Fernandez, F.G.; Molina Grima, E. Cultivation and anaerobic digestion of Scenedesmus spp. grown in a pilot-scale open raceway. Algal Res. 2014, 5, 95–102. [Google Scholar] [CrossRef]
- Hirayama, S.; Ueda, R.; Sugata, K. Evaluation of active oxygen effect on photosynthesis of Chlorella vulgaris. Free Rad. Res. 1996, 25, 247–254. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Novel outdoor thin-Iayer high density microalgal culture system: Productivity and operational parameters. Algol. Stud. 1995, 76, 129–147. [Google Scholar]
- Gonçalves, A.L.; Simões, M.; Pires, J.C.M. The effect of light supply on microalgal growth, CO2 uptake and nutrient removal from wastewater. Energy Conv. Manag. 2014, 85, 530–536. [Google Scholar] [CrossRef]
- Gouveia, L. Microalgae as a Feedstock for Biofuels; Springer: Heidelberg, Germany, 2011; pp. 30–32. [Google Scholar]
- Wang, B.; Lan, C.Q. Biofixation of carbon dioxide (CO2) by microorganisms. In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology, 1st ed.; Maroto-Valer, M.M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; Volume 2. [Google Scholar]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Vandamme, D. Flocculation Based Harvesting Processes for Microalgae Biomass Production. Ph.D. Thesis, KU Leuven, Belgium, May 2013. [Google Scholar]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Directive 1991/271/EEC. Directive of the European Council of 21 May 1991 Concerning Urban Waste-Water Treatment. Off. J. Eur. Union 1991, L 135, 40–52. [Google Scholar]
- Directive 1998/15/EC. Directive of the European Comission of 27 February 1998 amending Council Directive 91/271/EEC with respect to certain requirements established in Annex I thereof. Off. J. Eur. Union 1998, L 67, 29–30. [Google Scholar]
- Guderjan, M.; Töpfl, S.; Angersbach, A.; Knorr, D. Impact of pulsed electric field treatment on the recovery and quality of plant oils. J. Food Eng. 2005, 67, 281–287. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqui, A.H.; Haik, Y.; Farid, M.M. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Guderjan, M.; Elez-Martínez, P.; Knorr, D. Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 55–62. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Foltz, G. Algae Lysis with Pulsed Electric Fields. Master’s Thesis, California State Polytechnic University, San Luis Obispo, CA, USA, May 2012. [Google Scholar]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Schlagermann, P.; Göttlicher, G.; Dillschneider, R.; Rosello-Sastre, R.; Posten, C. Composition of algal oil and its potential as biofuel. J. Comb. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.M.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production; Energy Biosciences Institute: Berkeley, CA, USA, 2010. [Google Scholar]
- Fans—Efficiency and Power Consumption. Available online: http://www.engineeringtoolbox.com/fans-efficiency-power-consumption-d_197.html (accessed on 22 December 2015).
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Chini Zittelli, G.; Sampietro, G. Energy balance of algal biomass production in a 1-ha “Green Wall Panel” plant: How to produce algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Biogas renewable energy—The biogas composition. Available online: http://www.biogas-renewable-energy.info/biogas_composition.html (accessed on 22 December 2015).
- Davis, R.; Fishman, D.; Frank, E.D.; Wigmosta, M.S. Renewable Diesel from Algal Lipids: An Integrated Baseline for Cost, Emissions, and Resource Potential from a Harmonized Model; U.S. Department of Energy Biomass Program: Oak Ridge, TN, USA, 2012. [Google Scholar]
- Acién, F.; Fernández, J.; Magán, J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Benemann, J.R.; Oswald, W.J. Systems and Economic Analysis of Microalgae Ponds for Conversion of CO2 to Biomass—Final Report; Department of Civil Engineering of the California University: Berkeley, CA, USA, 1996. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D. Plant. Design and Economics for Chemical Engineers, 4th ed.; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Sinnott, R.K.; Towler, G. Chemical Engineering Design, 4th ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 2009; pp. 243–268. [Google Scholar]
- Chemical Market Insight and Foresight on a Single Page—Caustic Soda. Available online: http://www.orbichem.com/userfiles/CNF%20Samples/cas_13_11.pdf (accessed on 22 Feburay 2016).
- FAQ about Pulsed Electric Field Processing. Available online: https://www.pulsemaster.us/pef-pulsemaster/faq (accessed on 23 December 2015).
- Declercq, B.; Delarue, E.; D’haeseleer, W. Impact of the economic recession on the European power sector’s CO2 emissions. Energy Policy 2011, 39, 1677–1686. [Google Scholar] [CrossRef]
- Mithá, O. Análise de Projectos de Investimento; Escolar Editora: Lisboa, Portugal, 2004. (In Portuguese) [Google Scholar]





| Scenarios‘ Assumptions | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| Photosynthetic efficiency (%) | 2 | 1 | 3 | 2 | 2 | 2 | 2 |
| Biomass productivity (g·m−2·day−1) | 16.4 | 8.2 | 24.6 | 16.4 | 16.4 | 16.4 | 16.4 |
| Lipids extraction efficiency (%) | 75 | 75 | 75 | 60 | 90 | 75 | 75 |
| Anaerobic digestion efficiency (%) | 45 | 45 | 45 | 45 | 45 | 30 | 60 |
| Streams | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| —water input in the cultivation step (×104 m3·day−1) | 3.5 | 1.9 | 5.2 | 3.5 | 3.5 | 3.5 | 3.5 |
| —biomass flow rate after the cultivation step a (×104 m3·day−1) | 3.3 | 1.6 | 4.9 | 3.3 | 3.3 | 3.3 | 3.3 |
| —biomass flow rate after the pre-concentration step (×103 m3·day−1) | 8.2 | 4.1 | 12 | 8.2 | 8.2 | 8.2 | 8.2 |
| —biomass flow rate after the centrifugation step (m3·day−1) | 78 | 39 | 117 | 78 | 78 | 78 | 78 |
| —extracted lipids flow rate (m3·day−1) | 3.4 | 1.7 | 5.1 | 2.7 | 4.1 | 3.4 | 3.4 |
| —biomass flow rate after the lipids extraction step (m3·day−1) | 75 | 37 | 112 | 75 | 74 | 75 | 75 |
| —biogas flow rate after the anaerobic digestion step (t·day−1) | 6.6 | 3.3 | 10 | 6.9 | 6.3 | 4.4 | 8.9 |
| —flow rate of the residue produced in the anaerobic digestion step (t·day−1) | 7.0 | 3.5 | 11 | 7.3 | 6.6 | 8.9 | 5.1 |
| —water flow rate required to compensate evaporation losses (×103 m3·day−1) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| —wastewater flow rate required to feed the culture (×104 m3·day−1) | 2.4 | 0.62 | 5.2 | 2.4 | 2.4 | 2.4 | 2.4 |
| —recycling water flow rate required to feed the culture (×104 m3·day−1) | 1.1 | 1.3 | 0 | 1.1 | 1.1 | 1.1 | 1.1 |
| —anaerobic digestion effluent flow rate required to feed the culture (m3·day−1) | 62 | 31 | 93 | 62 | 62 | 62 | 62 |
| Nutrients Loads and Removal Rates | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| (g·m−2·day−1) | 1.1 | 0.54 | 1.6 | 1.1 | 1.1 | 1.1 | 1.1 |
| (mg·L−1) | 31 | 15 | 46 | 31 | 31 | 31 | 31 |
| (g·m−2·day−1) | 0.22 | 0.11 | 0.33 | 0.22 | 0.22 | 0.22 | 0.22 |
| (mg·L−1) | 6.2 | 3.1 | 9.2 | 6.2 | 6.2 | 6.2 | 6.2 |
| (g·m−2·day−1) | 8.4 | 4.2 | 13 | 8.4 | 8.4 | 8.4 | 8.4 |
| (t·day−1) | 39 | 19 | 58 | 39 | 39 | 39 | 39 |
| CO2 Streams | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| CO2 required for microalgal growth (t·day−1) | 39 | 19 | 58 | 39 | 39 | 39 | 39 |
| CO2 resulting from the anaerobic digestion (t·day−1) | 4.3 | 2.1 | 6.4 | 4.5 | 4.1 | 2.9 | 5.7 |
| CO2 resulting from CHP generation (t·day−1) | 6.4 | 3.2 | 9.7 | 6.7 | 6.1 | 4.3 | 8.6 |
| Net CO2 balance (t·day−1) | −20 | −10 | −30 | −20 | −21 | −24 | −17 |
| Energetic Streams | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| Energy required in microalgal cultivation (×103 kWh·day−1) | 6.2 | 5.3 | 7.0 | 6.2 | 6.2 | 6.2 | 6.2 |
| Energy required in microalgal harvesting (×103 kWh·day−1) | 9.8 | 4.9 | 15 | 9.8 | 9.8 | 9.8 | 9.8 |
| Energy required in cell disruption and lipids extraction (×103 kWh·day−1) | 7.5 | 3.7 | 11 | 7.5 | 7.5 | 7.5 | 7.5 |
| Energy obtained from the extracted lipids (×104 kWh·day−1) | 3.0 | 1.5 | 4.6 | 2.4 | 3.6 | 3.0 | 3.0 |
| Electrical energy produced in the CHP generation unit (×104 kWh·day−1) | 1.4 | 0.69 | 2.1 | 1.4 | 1.3 | 0.92 | 1.8 |
| Thermal energy produced in the CHP generation unit (×104 kWh·day−1) | 1.6 | 0.77 | 2.3 | 1.6 | 1.5 | 1.0 | 2.1 |
| EROEI | 2.5 | 2.1 | 2.7 | 2.3 | 2.7 | 2.1 | 3.0 |
| Equipments | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| High rate pond a | 3479 | 3479 | 3479 | 3479 | 3479 | 3479 | 3479 |
| Air blowers b | 123 | 62 | 185 | 123 | 123 | 123 | 123 |
| Clarifier c | 325 | 163 | 488 | 325 | 325 | 325 | 325 |
| Centrifuge d | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Decanter e | 44 | 44 | 89 | 44 | 44 | 44 | 44 |
| Digester and CHP generation unit f | 1399 | 1399 | 1399 | 1399 | 1399 | 1399 | 1399 |
| Total | 5753 | 5530 | 6023 | 5753 | 5753 | 5753 | 5753 |
| Total purchase costs | 5983 | 5734 | 6281 | 5983 | 5983 | 5983 | 5983 |
| Costs | Factor a | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|---|
| Direct costs | ||||||||
| Total purchase costs | 1.00 | 5983 | 5734 | 6281 | 5983 | 5983 | 5983 | 5983 |
| Purchased equipment installation | 0.20 | 1197 | 1147 | 1256 | 1197 | 1197 | 1197 | 1197 |
| Instrumentation and control | 0.15 | 897 | 860 | 942 | 897 | 897 | 897 | 897 |
| Piping | 0.20 | 1197 | 1147 | 1256 | 1197 | 1197 | 1197 | 1197 |
| Electrical | 0.10 | 598 | 573 | 628 | 598 | 598 | 598 | 598 |
| Buildings | 0.15 | 897 | 860 | 942 | 897 | 897 | 897 | 897 |
| Yard improvements | 0.05 | 299 | 287 | 314 | 299 | 299 | 299 | 299 |
| Service facilities | 0.20 | 1197 | 1147 | 1256 | 1197 | 1197 | 1197 | 1197 |
| Indirect costs | ||||||||
| Engineering and supervision | 0.3 | 1795 | 1720 | 1884 | 1795 | 1795 | 1795 | 1795 |
| Construction expenses | 0.05 | 299 | 287 | 314 | 299 | 299 | 299 | 299 |
| Contractor’s fee | 0.03 | 179 | 172 | 188 | 179 | 179 | 179 | 179 |
| Contingency | 0.08 | 479 | 459 | 502 | 479 | 479 | 479 | 479 |
| Total capital cost | 15,017 | 14,391 | 15,765 | 15,017 | 15,017 | 15,017 | 15,017 |
| Costs | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| Variable costs | |||||||
| Raw materials | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Miscellaneous materials a | 75 | 74 | 77 | 75 | 75 | 75 | 75 |
| Utilities | 857 | 510 | 1205 | 857 | 857 | 857 | 857 |
| Pre-concentration with NaOH | 35 | 17 | 52 | 35 | 35 | 35 | 35 |
| PEF extraction | 11 | 5 | 16 | 9 | 13 | 11 | 11 |
| Shipping and packaging b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fixed costs | |||||||
| Maintenance c | 751 | 738 | 766 | 751 | 751 | 751 | 751 |
| Operating labour | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
| Laboratory costs d | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Supervision d | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Plant overheads e | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Insurance f | 150 | 148 | 153 | 150 | 150 | 150 | 150 |
| Local taxes g | 300 | 295 | 306 | 300 | 300 | 300 | 300 |
| Royalties f | 150 | 148 | 153 | 150 | 179150 | 150 | 150 |
| Annual production costs | 2557 | 2164 | 2956 | 2555 | 2559 | 2557 | 2557 |
| Economic Viability Parameters | Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Sc7 |
|---|---|---|---|---|---|---|---|
| Interest rate (%) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Lifetime (years) | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Net present value (NPV, k€) | 5287 | −12,124 | 22,609 | 4267 | 6307 | 4940 | 5634 |
| Internal rate of return (IRR, %) | 14 | n.a. | 26 | 13 | 15 | 14 | 14 |
| Payback time (years) | 8 | n.a. | 4 | 8 | 7 | 8 | 8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.L.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Simões, M.; Pires, J.C.M. Integration of Microalgae-Based Bioenergy Production into a Petrochemical Complex: Techno-Economic Assessment. Energies 2016, 9, 224. https://doi.org/10.3390/en9040224
Gonçalves AL, Alvim-Ferraz MCM, Martins FG, Simões M, Pires JCM. Integration of Microalgae-Based Bioenergy Production into a Petrochemical Complex: Techno-Economic Assessment. Energies. 2016; 9(4):224. https://doi.org/10.3390/en9040224
Chicago/Turabian StyleGonçalves, Ana L., Maria C. M. Alvim-Ferraz, Fernando G. Martins, Manuel Simões, and José C. M. Pires. 2016. "Integration of Microalgae-Based Bioenergy Production into a Petrochemical Complex: Techno-Economic Assessment" Energies 9, no. 4: 224. https://doi.org/10.3390/en9040224
APA StyleGonçalves, A. L., Alvim-Ferraz, M. C. M., Martins, F. G., Simões, M., & Pires, J. C. M. (2016). Integration of Microalgae-Based Bioenergy Production into a Petrochemical Complex: Techno-Economic Assessment. Energies, 9(4), 224. https://doi.org/10.3390/en9040224