3. Results and Discussion
The XRD patterns of Dy
2O
3-TiO
2 powder mixtures milled at 500 rpm and 200 rpm for different times are shown in
Figure 1. The XRD results show that the crystal structure of the original Dy
2O
3 phase and TiO
2 phase are cubic and rutile, respectively. At the condition of 500 rpm, the diffraction peaks of cubic Dy
2O
3 and TiO
2 broadened significantly and reduced in intensity with increased milling time. The broadening of the X-ray diffraction peaks is associated with the refinement in grain size and lattice distortions. Meanwhile, the diffraction peaks of monoclinic Dy
2O
3 can be observed in the X-ray patterns as indicated by the black inverted triangles in
Figure 1a. Ball milling induces a Dy
2O
3 phase transformation from the cubic to the monoclinic crystal structure. A broad, singular diffraction peak is also present that indicates the formation of the amorphous phase during ball milling. Interestingly, the amorphous peak is present at the location of the diffraction peak for the monoclinic Dy
2O
3 phase, not at the location of the diffraction peak for the cubic Dy
2O
3 phase, which indicates that the formed amorphous phase is derived from the monoclinic Dy
2O
3 phase, not from the cubic Dy
2O
3 phase. The transformation from cubic to monoclinic increases with increased milling time. After milling for 96 h, only the amorphous phase can be observed, which indicates that the monoclinic Dy
2O
3 phase was fully converted to the amorphous phase. Additionally, this behavior indicates that no new compounds are synthesized during ball milling. Even if new compounds were formed in the milled powders, the amount is very low and does not reach the sensitivity range of the X-ray measurement. Therefore, in the present work, the evolution of Dy
2O
3-TiO
2 powder mixtures is as follows: ball milling first induces the transformation of Dy
2O
3 from the cubic to the monoclinic crystal phase, then monoclinic Dy
2O
3 undergoes amorphization, and finally the powder mixtures completely transform to the amorphous phase.
However, the ball-milling behavior of powder mixtures at 200 rpm is distinctly different from that at 500 rpm. The change of the diffraction peaks with increased milling time at 200 rpm is shown in
Figure 1b. Although the diffraction peaks of cubic Dy
2O
3 and TiO
2 are also broadened and reduced in intensity with increased milling time, the diffraction peaks of TiO
2 can be observed in the XRD spectrums and are not disappeared. After ball milling for 96 h, the intensity of diffraction peaks of Dy
2O
3 and TiO
2 are also high. The powder mixtures are not changed completely to amorphization. According to the shape of XRD diffraction spectrums, the effect of ball milling on powder mixtures after milling for 96 h at 200 rpm is only similar to that after milling for 4 h at 500 rpm. Therefore, at low ball-milling rotation speeds, the powder mixtures are only fined and homogenized.
Our experimental results are different from the results of G. Garcia-Martinez [
7]. In their research, ball milling induced a phase transformation in Dy
2O
3 from cubic to monoclinic. However, a Dy
2TiO
5 compound with a hexagonal crystal structure was formed simultaneously. The ball-milled powders consisted of mixed phases of hexagonal Dy
2TiO
5 and monoclinic Dy
2O
3. The Dy
2O
3-TiO
2 powder mixtures did not completely transform to the amorphous phase, but instead produced the hexagonal Dy
2TiO
5 phase. This difference can be attributed to the different ball-milling conditions used in this research, with special attention to the different ball-milling facilities. During ball milling, experimental parameters such as rotation speed, ball-milling time, ball-milling media, and the ball-to-powder mass ratio have an important influence on the ball-milled products even when using the same proportion and type of oxides. For example, Gajović reported that nanosized ZrTiO
4 formed in ZrO
2-TiO
2 powder mixtures, whereas only amorphous mixtures were obtained during ball milling in Stubičar’s work [
21,
22]. In addition, the polymorphic transformation of Ln
2O
3 was also observed in Gd
2O
3-TiO
2 and Y
2O
3-2TiO
2 powder systems [
23].
The variation of Dy
2O
3 grain size with ball-milling time at the rotational speeds of 500 rpm and 200 rpm is shown in
Figure 2. Actually, the size of Dy
2O
3 grain was calculated for Dy
2O
3 with cubic structure, not for Dy
2O
3 with monoclinic structure, because ball milling induced a phase transformation in Dy
2O
3 from cubic to monoclinic and simultaneously from monoclinic to amorphous. It is difficult to calculate the grain size of Dy
2O
3 with monoclinic structure. It can be seen that ball milling results in a fast decrease of Dy
2O
3 grain size in the initial stage at both 500 rpm and 200 rpm. The refinement rate of crystallite size is roughly logarithmic with ball-milling time at 200 rpm. After 96 h of ball milling, the size of Dy
2O
3 grain is up to approximately 60 nm. However, at 500 rpm, the diffraction peaks of Dy
2O
3 with cubic structure are hardly observed in the XRD spectrum after milling for 24 h as shown in
Figure 1a, especially, when the ball-milling time is over 48 h. Therefore, the size of Dy
2O
3 with cubic structure is calculated only before 12 h of ball-milling time. It can be seen that the grain size is quickly decreased. In addition, after same ball-milling time, the grain size of Dy
2O
3 phase at the 500 rpm is obviously smaller than that at the 200 rpm. The effect of ball milling on the grain refinement of powder mixtures at 500 rpm is significantly more intense than that at 200 rpm. The size of Dy
2O
3 grain in the powder mixtures after milling for 4 h at 500 rpm is about 52 nm, which is smaller than that after milling for 96 h at 200 rpm (approximately 60 nm).
The morphology evolution of Dy
2O
3-TiO
2 powder mixtures with increasing ball-milling time at 500 rpm is shown in
Figure 3. Both TiO
2 and Dy
2O
3 are brittle components, which are fragmented during ball milling and particle size reduces continuously as a consequence of the energy provided during ball milling. The morphology of large particles is changed significantly due to fracture, agglomeration, and deagglomeration processes. The morphology of the original powder mixtures consists of large-sized Dy
2O
3 particles in micrometer size and small-sized TiO
2 particles in nanometer. The shape of the powder particles is irregular. The line-scanning results of elemental Dy, Ti, and O in the characteristic position in
Figure 3a are shown in
Figure 3b and also inserted in
Figure 3a. It can be seen that the small-sized particles are TiO
2 component and the large-sized particles are Dy
2O
3 components from the variation of the elemental diffraction intensity. The brittle Dy
2O
3 particles are fragmented by ball-powder-ball collisions, leading to a considerable reduction in the powder particle size and subsequent amorphization as milling time increases. After ball milling for 4 h, the size of particles decreases significantly. A large number of small size of Dy
2O
3 particles in nanometer can be observed in the milled powders as shown in
Figure 3c. The morphology of the powder mixtures is transformed to uniform, as shown in
Figure 3c–g, where the ball-milling time ranges from 4 to 96 h. The morphologies demonstrate that the refining effects of the powder particles are proportional to the ball-milling time for the same rotational speed. After 96 h of ball milling, a large number of nanoparticles agglomerate to form a large-sized particle, as shown in
Figure 3g. In addition, TiO
2 particles disappear after ball milling for 96 h, as shown in
Figure 1a. The surfaces of the Dy
2O
3 particles in
Figure 3g are clean compared with those in
Figure 3a. It can be concluded that the particle size in the powder mixtures is refined to the nanoscale after ball milling for 96 h. The line-scanning results of elemental Dy, Ti, and O in the characteristic position in
Figure 3g is shown in
Figure 3h and also inserted in
Figure 3g, which indicates these elements are uniformly distributed in the ball-milled particles according the variation of the elemental diffraction intensity. In addition, the morphology evolution of powder mixtures at an 200 rpm dose are not provided in the present work because the powder mixtures are only fined and homogenized according to the XRD results as shown in
Figure 1b. The morphology of mixtures after ball milling for 96 h at 200 rpm is similar with that after ball milling for 4 h at 500 rpm.
Figure 4 shows TEM images and corresponding selected area electron diffraction (SAED) patterns of Dy
2O
3-TiO
2 powder mixtures milled for 4 h and 96 h. After milling for 4 h, nano-sized ball-milled powder particles aggregate to form large-sized particles, as shown in
Figure 4a, due to the high active surface energy created upon ball milling. The size of the original TiO
2 particles is approximately 50 nm. From the XRD results, it can be observed that ball milling leads to TiO
2 particle refinement, dissolution, and finally disappearance after 96 h. Therefore, the main particles presented in the TEM image are Dy
2O
3. The bright zones near the edge of the powder particles are the thin areas where the electron beam penetrates. The dark areas in the images of the powder particles are the thick areas where the electron beam rarely penetrates. Indexing and analyzing the ring-shaped SAED pattern taken from the area denoted by the letter “A” indicates that the Dy
2O
3 grains are already nanocrystalline. The diffraction spots coming from cubic Dy
2O
3, monoclinic Dy
2O
3, and TiO
2 grains are present in the SAED pattern in
Figure 4b. The diffraction halo in the SAED pattern also indicates the formation of an amorphous phase during high-energy ball milling.
After ball milling for 96 h, the ball-milled powders agglomerate to form large particles with large thicknesses such that the electron beam rarely penetrates, showing as a dark color in the TEM image in
Figure 4c. It is difficult to observe the microstructure of the agglomerated particles. The SAED pattern from the area marked with the letter “B” indicates that the powder mixtures are almost completely converted to the amorphous phase, although sporadic diffraction spots are also present in this pattern. The atom arrangement in the amorphous phase is disordered over long distances, but ordered over short distances. As grain size decreases, the number of atoms at the grain boundaries increases. The proportion of atoms in the crystal volume relative to the crystal boundary decreases. Schwarz and Koch noted that the formation of an amorphous phase in as-milled powders was similar to the amorphization that occurs during the isothermal annealing of crystalline metallic thin films [
24]. In high-energy ball milling, the intense deformation accelerates interdiffusion, and the large defect density increases the free energy of the components in the mixture to form an amorphous product.
Monoclinic Ln
2O
3 is initially formed in the mechanical alloying of lanthanum titanate or dititanate. In Moreno’s research, the formation of Gd
2(Ti
(1−y)Zr
y)
2O
7 pyrochlores occurred in the final step of ball milling starting from an amorphous matrix of Gd
2O
3, TiO
2, and ZrO
2 [
23]. However, in the present work, after 96 h of ball milling, the monoclinic Dy
2O
3 phase could not be detected and was completely transformed to the amorphous phase. Moreover, dysprosium titanate also could not be detected. To investigate the sintering behavior of the ball-milled powder mixtures, DSC was carried out on the powder mixtures milled for 96 h at test temperatures ranging from 200 to 1200 °C. The DSC curve in
Figure 5 shows one exothermic peak close to 880 °C and one endothermic peak close to 1145 °C. An exothermic peak in a DSC curve can generally be attributed to a transition from disordered to ordered, the recrystallization of original components from the amorphous phase or the formation of a new compound from the ball-milled amorphous powders. Therefore, subsequent X-ray analysis of the ball-milled powders annealed at different temperatures is used to further analyze occurrences in the heating process in more detail. The powder mixtures transform completely to the amorphous state after ball milling for 96 h. Therefore, it seems feasible that the transition from disordered to ordered produced the exothermic peak in the DSC curve. In fact, only the amorphous peak is observed in the XRD pattern of the 96 h ball-milled powder after annealing for 24 h at 700 °C. No diffraction peaks for Dy
2O
3 or TiO
2 are detected. Even with prolonged annealing time, the XRD results are the same. Therefore, the exothermic peak in the DSC curve should be related to the new phase generated from the amorphous mixtures.
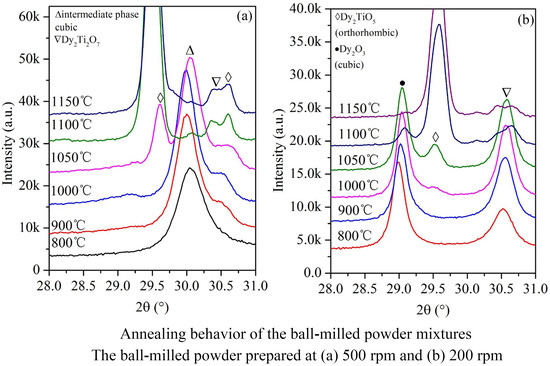
The powder mixtures milled for 96 h were annealed for 3 h at 800, 900, 1000, 1050, 1100, and 1150 °C. The XRD results of the annealed powder mixtures are shown in
Figure 6a,b. Several diffraction peaks different from the diffraction peaks of Dy
2O
3 (cubic and monoclinic crystal structure) and TiO
2 (rutile structure) are observed, which indicates new components with crystal structures generated from the amorphous mixtures. This experimental phenomenon of synthesizing new compounds is similar to Stubičar’s research in which the orthorhombic ZrTiO
4 phase was generated from the high-temperature annealing of amorphous mixtures formed from the ball milling of a ZrO
2-TiO
2 powder system [
21] and consistent with Khor’s results that zirconia was produced from annealing amorphous mixtures formed from the ball milling of an equimolar ZrSiO
4 and Al
2O
3 powder system [
25].
According to the XRD standard database, cubic Dy
2TiO
5 {111} presents at 2θ = 30.043°, hexagonal Dy
2TiO
5 {102} presents at 2θ = 32.411°, orthorhombic Dy
2TiO
5 {201} presents at 2θ = 29.554°, and pyrochlore Dy
2Ti
2O
7 {111} presents at 2θ = 30.698°; the difference in the above diffraction angles is not very large. Three diffraction peaks are observed in the XRD patterns of the powder mixtures annealed for 3 h at 800, 900, and 1000 °C. The main diffraction peak representing the crystalline phase is at 2θ = 30.0°, as shown in
Figure 6b. Therefore, the newly formed product in the powder system annealed between 800 °C and 1000 °C is not pyrochlore Dy
2Ti
2O
7. This result is different than that found in Amit Sinha’s research in which pyrochlore Dy
2Ti
2O
7 was initially created in the formation of Dy
2TiO
5 [
19]. In their research using an equimolar Dy
2O
3-TiO
2 system, the chemical reaction of Dy
2O
3 and TiO
2 initially formed pyrochlore Dy
2Ti
2O
7, and then Dy
2Ti
2O
7 reacted with the remaining Dy
2O
3 to form orthorhombic Dy
2TiO
5.
According to the equilibrium phase diagram, the Dy
2TiO
5 phase has three crystal structure types depending on the temperature: orthorhombic
hexagonal
cubic [
15]. Transformation from the high-temperature phase to the low-temperature phase is an exothermic process. For example, the polymorphic transformation of Gd
2TiO
5 from hexagonal to orthorhombic produced an exothermic peak in the DSC curve [
7]. However, in the present work, there is only one exothermic peak in the DSC curve. Although the diffraction peaks in the XRD patterns match well with the diffraction peaks in the standard pattern of cubic Dy
2TiO
5, the generated phase should not be cubic Dy
2TiO
5 because low-temperature phases of orthorhombic and hexagonal Dy
2TiO
5 were not detected after annealing over temperatures ranging from 700 to 1000 °C for annealing times ranging from several minutes to 3 h; additionally, as mentioned above, the formation temperature of cubic Dy
2TiO
5 is over 1680 °C. It is not possible to achieve such a high temperature during ball milling. Therefore, the high-temperature phase of cubic Dy
2TiO
5 should not be produced. Instead, the generated phase is an intermediate phase that has a similar crystal structure to cubic Dy
2TiO
5 and is a metastable state that phase transforms to orthorhombic Dy
2TiO
5 when the annealing temperature is above 1050 °C. After annealing for 3 h at 1100 °C, orthorhombic Dy
2TiO
5 and a small amount of pyrochlore Dy
2Ti
2O
7 and cubic Dy
2O
3 are detected; notably, cubic Dy
2TiO
5 is not observed in the ball-milled powder mixtures.
The change of the grain size of the main characteristic phase in the annealed powder mixtures with annealing temperature is shown in
Figure 6c. According to the above results, the grain size of the intermediate phase and orthorhombic Dy
2TiO
5 are calculated using Suryanarayana and Grant Norton’s formula. It can be seen that the grain size increases with increasing annealing temperature. The grain size of the intermediate phase is about 27 nm in the powder mixtures annealed for 3 h at 800 °C and is about 275 nm in the powder mixtures annealed for 3 h at 1050 °C. Because the intermediate phase transforms to orthorhombic Dy
2TiO
5 when the annealing temperature is above 1050 °C, the grain size of orthorhombic Dy
2TiO
5 is calculated at the annealing temperature ranging from 1050 °C to 1150 °C. The grain size of orthorhombic Dy
2TiO
5 is about 43 and 380 nm after annealing for 3 h at 1050 °C and 1150 °C, respectively.
In addition, the pressure created during ball milling is not high enough to transform the crystal structure of Dy
2TiO
5 from orthorhombic to hexagonal or from hexagonal to cubic. The average pressure on the contact surface of two colliding mill balls is approximately 8.5 GPa [
26], which is far below the 100 GPa needed to induce a pressure wave to cause the Gd
2TiO
5 phase transformation from the low-temperature orthorhombic phase to the high-temperature hexagonal phase [
27]. Therefore, the intermediate phase is not produced by collision pressure. Further investigation is needed into the cause of the formation of the intermediate phase.
To further investigate the annealing behavior of the ball-milled powder mixtures, the powder mixtures milled for 96 h at 200 rpm were sintered for 3 h at 800, 900, 1000, 1050, 1100, and 1150 °C. The phase evolution of the annealed powder mixtures identified by XRD analysis is shown in
Figure 7. Under these ball-milling conditions, the Dy
2O
3-TiO
2 powder mixtures are homogenized, and the polymorphic transformation of Dy
2O
3 from cubic to monoclinic is not observed in
Figure 7a. In the XRD pattern of the powder mixtures annealed for 3 h at 1000 °C, diffraction peaks for Dy
2O
3 and Dy
2Ti
2O
7 phase are observed. However, the main diffraction peak for orthorhombic Dy
2TiO
5 is not detected. Under these conditions, the annealed powder mixtures are composed of cubic Dy
2O
3 and pyrochlore Dy
2Ti
2O
7. The powder mixtures generate the orthorhombic Dy
2TiO
5 phase at 1050 °C and are composed of cubic Dy
2O
3, pyrochlore Dy
2Ti
2O
7, and orthorhombic Dy
2TiO
5. The powder mixtures are almost completely transformed to orthorhombic Dy
2TiO
5 after annealing at 1150 °C for 3 h. The diffraction peak intensity of orthorhombic Dy
2TiO
5 gradually increases with increasing annealing temperature, and simultaneously, the diffraction peak intensity of Dy
2O
3 and Dy
2Ti
2O
7 decreases with increasing annealing temperature. For powder mixtures ball milled for 96 h at 200 rpm, the evolutionary behavior at various annealing temperatures is consistent with the data presented in Ref. [
19], in which Amit Sinha reported that the chemical reaction of Dy
2O
3 and TiO
2 initially formed pyrochlore Dy
2Ti
2O
7, and then Dy
2Ti
2O
7 reacted with the remaining Dy
2O
3 to form orthorhombic Dy
2TiO
5. However, this experimental phenomenon is different from that observed in the annealed powder mixtures milled for 96 h at 500 rpm, as shown in
Figure 6, due to the initial conditions of the ball-milling mixtures.
The change of the grain size of main characteristic phase in the annealed powder mixtures with annealing temperature is shown in
Figure 7c. The grain size of the cubic Dy
2O
3, pyrochlore Dy
2Ti
2O
7 and orthorhombic Dy
2TiO
5 are calculated using Suryanarayana and Grant Norton’s formula. The grain size of cubic Dy
2O
3 and pyrochlore Dy
2Ti
2O
7 increases with increasing annealing temperature. Because the orthorhombic Dy
2TiO
5 is detected in the powder mixtures annealed at 1050 °C for 3 h, the grain size of orthorhombic Dy
2TiO
5 is calculated when the annealing temperature is over 1050 °C. The grain size of orthorhombic Dy
2TiO
5 is about 38 nm and 395 nm after annealing 3 h at 1050 °C and 1150 °C, respectively.
Figure 8a,b shows the bright field TEM image and corresponding SAED pattern of the ball-milled Dy
2O
3-TiO
2 powder mixtures annealed at 1000 °C for 3 h, respectively. The powder mixtures are previously milled for 96 h at 500 rpm. After annealing for 3 h, the grain size of powder mixtures is kept in nanometer scale, which can be supported by the corresponding SAED pattern taken from the region marked letter “A” in
Figure 8a. The diffraction ring is a typical SAED pattern of nanocrystal materials. After analyzing and indexing the ring-shaped SAED pattern, it is indicated that this SAED pattern belongs to the intermediate Dy
2TiO
5 phase that has a similar crystal structure to cubic Dy
2TiO
5, which is in accord with the XRD results as shown in
Figure 6. The small-sized powders agglomerate to form large particles with large thicknesses as shown in
Figure 8a. The bright zones near the edge of the powder particles is the thin area where the electron beam penetrates. The dark area in the image of the powder particles is the thick area where the electron beam rarely penetrates. After annealing, the amorphous ball-milled powder mixtures are changed to the intermediate Dy
2TiO
5 phase with crystal structure.
Figure 8c,d is the bright field TEM image and corresponding SAED pattern of the annealed Dy
2O
3-TiO
2 powder mixtures that were previously milled for 96 h at 200 rpm. Indexing and analyzing the ring-shaped SAED pattern taken from the area denoted by the letter “B” indicates that the particles are composed of cubic Dy
2O
3 and pyrochlore Dy
2Ti
2O
7, which is accord with the XRD results of the powder mixtures annealed at 1000 °C for 3 h as shown in
Figure 7. This experimental result is different from that observed in the annealed powder mixtures milled for 96 h at 500 rpm.