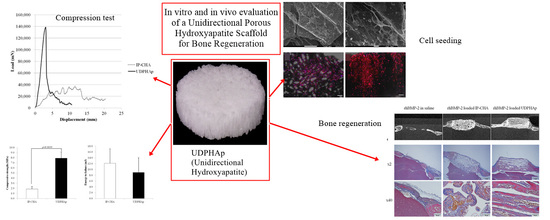
Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Scaffolds
2.3. Scaffold Characterization by Micro-CT before Cell Seeding
2.4. Calculation of Porosity and SSA
2.5. Strength Test
2.6. In Vitro rhBMP-2 Releasing Assay
2.7. Cell Culture and Seeding Cells onto the Scaffold
2.8. Observation of Cells Attached to the Scaffolds’ Surface by a Scanning Electron Microscope
2.9. Observation of Cell Adhesion by Fluorescence Microscopy
2.10. Seeding Efficiency Analysis
2.11. Evaluation of Attached Cell Number
2.12. Healing of Critical-Size Mouse Calvarial Bone Defects in Combination with rhBMP-2
2.13. Statistical Analysis
3. Results
3.1. Scaffold Characterization by Micro-CT before Cell Seeding
3.2. Strength Test
3.3. In Vitro rhBMP-2 Releasing Assay
3.4. Observation of Cells Attached to the Scaffolds’ Surface by a Scanning Electron Microscope
3.5. Observation of Cell Adhesion by Fluorescence Microscopy
3.6. Seeding Efficiency Analysis
3.7. Evaluation of Attached Cell Number
3.8. Healing of Mouse Critical-Size Calvarial Bone Defects in Combination with rhBMP-2
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42 (Suppl. 2), S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Gentile, P.; Wilcock, C.; Miller, C.; Moorehead, R.; Hatton, P. Process optimisation to control the physico-chemical characteristics of biomimetic nanoscale hydroxyapatites prepared using wet chemical precipitation. Materials (Basel) 2015, 8, 2297–2310. [Google Scholar] [CrossRef]
- Liu, X.; Bao, C.; Xu, H.H.K.; Pan, J.; Hu, J.; Wang, P.; Luo, E. Osteoprotegerin gene-modified BMSCs with hydroxyapatite scaffold for treating critical-sized mandibular defects in ovariectomized osteoporotic rats. Acta Biomater. 2016, 42, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Surmeneva, M.; Nikityuk, P.; Hans, M.; Surmenev, R. Deposition of ultrathin nano-hydroxyapatite films on laser micro-textured titanium surfaces to prepare a multiscale surface topography for improved surfacewettability/energy. Materials (Basel) 2016, 9, 862. [Google Scholar] [CrossRef]
- Keeney, M.; Chung, M.T.; Zielins, E.R.; Paik, K.J.; McArdle, A.; Morrison, S.D.; Ransom, R.C.; Barbhaiya, N.; Atashroo, D.; Jacobson, G.; et al. Scaffold-mediated BMP-2 minicircle DNA delivery accelerated bone repair in a mouse critical-size calvarial defect model. J. Biomed. Mater. Res. A 2016, 104, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, W.I.; Osiris, W.G.; Mohamed, S.S.; Khalil, M.R. Reconstruction of resected mandibles using a hydroxyapatite veterinary bone graft. Biomaterials 1994, 15, 609–614. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Funayama, T.; Noguchi, H.; Tsukanishi, T.; Sakane, M. histological analysis of bone bonding and ingrowth into connected porous hydroxyapatite spacers in spinal surgery. Key Eng. Mater. 2012, 529–530, 309–312. [Google Scholar] [CrossRef]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.S.; Lee, C.K.; Hong, K.S.; Youn, H.J.; Ryu, H.S.; Chung, S.S.; Park, K.W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Iwasashi, M.; Muramatsu, T.; Sakane, M. Radiological and histological evaluation of regenos® which implanted in human radial fracture: A clinical case report. Key Eng. Mater. 2013, 529–530, 313–316. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Sakane, M.; Noguchi, H.; Funayama, T.; Naoyuki, O. Novel scaffold for bone tissue engineering: Unidirectional porous hydroxyapatite. In Bone Grafts: Procedures, Complications and Alternatives; Nova Science Publishers: New York, NY, USA, 2013; pp. 29–40. [Google Scholar]
- Van Lenthe, G.H.; Hagenmüller, H.; Bohner, M.; Hollister, S.J.; Meinel, L.; Müller, R. Nondestructive micro-computed tomography for biological imaging and quantification of scaffold-bone interaction in vivo. Biomaterials 2007, 28, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Kodama, H.A.; Amagai, Y.; Yamamoto, S.; Kasai, S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 1983, 96, 191–198. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.P.; Gauthier, M.; Lefebvre, L.P.; Tabrizian, M. Three-dimensional growth of differentiating MC3T3-E1 pre-osteoblasts on porous titanium scaffolds. Biomaterials 2005, 26, 7319–7328. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Yilgor, P.; Tuzlakoglu, K.; Reis, R.L.; Hasirci, N.; Hasirci, V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 2009, 30, 3551–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.H.; Yun, Y.P.; Kim, H.J.; Park, K.; Kim, S.E.; Song, H.R. Bone formation in a rat tibial defect model using carboxymethyl cellulose/BioC/bone morphogenic protein-2 hybrid materials. Biomed. Res. Int. 2014, 2014, 230152. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Soon, Y.M.; Shin, K.H.; Koh, Y.H.; Lee, J.H.; Choi, W.Y.; Kim, H.E. Fabrication and compressive strength of porous hydroxyapatite scaffolds with a functionally graded core/shell structure. J. Eur. Ceram. Soc. 2011, 31, 13–18. [Google Scholar] [CrossRef]
- Kho, D.; MacDonald, C.; Johnson, R.; Unsworth, C.P.; O’Carroll, S.J.; du Mez, E.; Angel, C.E.; Graham, E.S. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors 2015, 5, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Martínez-vázquez, F.J.; Perera, F.H.; Miranda, P.; Pajares, A.; Guiberteau, F. Improving the compressive strength of bioceramic robocast scaffolds by polymer infiltration. Acta Biomater. 2010, 6, 4361–4368. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qu, X.; Cui, W.; Bei, J.; Yu, F.; Lu, S.; Wang, S. Manufacturing and morphology structure of polylactide-type microtubules orientation-structured scaffolds. Biomaterials 2006, 27, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Bonzani, I.C.; George, J.H.; Stevens, M.M. Novel materials for bone and cartilage regeneration. Curr. Opin. Chem. Biol. 2006, 10, 568–575. [Google Scholar] [CrossRef] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Aoki, K.; Okamoto, M.; Kato, H.; Saito, N. Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration. Materials 2017, 10, 33. https://doi.org/10.3390/ma10010033
Tanaka M, Haniu H, Kamanaka T, Takizawa T, Sobajima A, Yoshida K, Aoki K, Okamoto M, Kato H, Saito N. Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration. Materials. 2017; 10(1):33. https://doi.org/10.3390/ma10010033
Chicago/Turabian StyleTanaka, Manabu, Hisao Haniu, Takayuki Kamanaka, Takashi Takizawa, Atsushi Sobajima, Kazushige Yoshida, Kaoru Aoki, Masanori Okamoto, Hiroyuki Kato, and Naoto Saito. 2017. "Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration" Materials 10, no. 1: 33. https://doi.org/10.3390/ma10010033
APA StyleTanaka, M., Haniu, H., Kamanaka, T., Takizawa, T., Sobajima, A., Yoshida, K., Aoki, K., Okamoto, M., Kato, H., & Saito, N. (2017). Physico-Chemical, In Vitro, and In Vivo Evaluation of a 3D Unidirectional Porous Hydroxyapatite Scaffold for Bone Regeneration. Materials, 10(1), 33. https://doi.org/10.3390/ma10010033