Effect of Fungal Deterioration on Physical and Mechanical Properties of Hemp and Flax Natural Fiber Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Selection and Preparation
2.2. Fungal Spore Suspension
2.3. Experimental Design
2.3.1. Physical Testing
2.3.2. Mechanical Testing
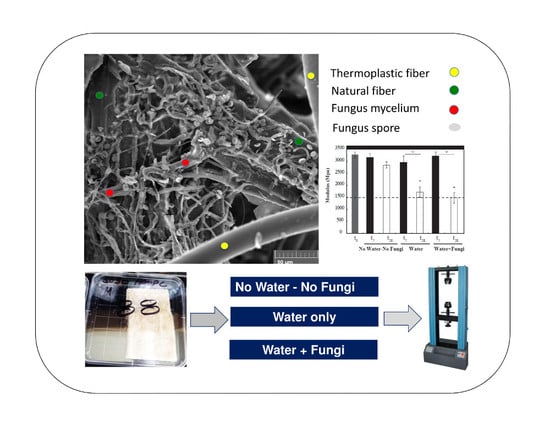
2.3.3. Scanning Electron Microscopy
3. Results
3.1. Physical Testing
3.2. Mechanical Testing
3.3. Scanning Electron Microscopy
4. Discussion: A Material Selection Perspective
5. Conclusions
- (i)
- Higher percentage of cellulose (less lignin) in the natural fibers; and
- (ii)
- Higher surface area (diameter and length) of the embedded natural fibers.
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Holmes, S. The 787 Encounters Turbulence: Technical Glitches and Manufacturing Woes could Delay Boeing’s Breakthrough. Business Week, 19 June 2006. [Google Scholar]
- Strong, A.B.; Ploskonka, C.A. Fundamentals of Composites Manufacturing: Materials, Methods and Applications; Society of Manufacturing Engineers, Publications Development Department: Dearborn, MI, USA, 1989. [Google Scholar]
- Boisse, P.; Hamila, N.; Vidal-Sallé, E.; Dumont, F. Simulation of wrinkling during textile composite reinforcement forming. Influence of tensile, in-plane shear and bending stiffnesses. Compos. Sci. Technol. 2001, 71, 683–692. [Google Scholar]
- Kazmierski, C. Growth Opportunities in Global Composites Industry, 2012–2017; Keynote Presentation of the Composites Exhibition and Convention: Las Vegas, NV, USA, 2012. [Google Scholar]
- Chattopadhyay, S.K.; Ghoshal, A.K.; Khandal, R.K.; Niyogi, U.K.; Pramanik, N.; Singh, S.; Uppaluri, R. Biodegradability studies on natural fibers reinforced polypropylene composites. J. Appl. Polym. sci. 2011, 121, 2226–2232. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzall, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Westman, M.P.; Fifield, L.S.; Simmons, K.L.; Laddha, S.G.; Kafentzis, T.A. Natural fiber-reinforced composites: A review. J. Polym. Environ. 2010, 15, 25–33. [Google Scholar]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Progress report on natural fiber reinforced composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Dhal, J.P.; Mishra, S.C. Processing and properties of natural fiber-reinforced polymer composite. J. Mater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Rong, M.Z.; Lu, X. Fully biodegradable natural fiber composites from renewable resources: All plant fiber composites. Compos. Sci. Technol. 2005, 65, 2514–2525. [Google Scholar] [CrossRef]
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- Alavudeen, A.; Thiruchitrambalam, M.; Venkateshwaran, N.; Athijayamani, A. Review of natural fiber reinforced woven composite. Rev. Adv. Mater. Sci. 2011, 27, 146–150. [Google Scholar]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Lucintel, B. Opportunities in Natural Fiber Composites. 2014. Available online: http://www.lucintel.com/OpportunitiesinNaturalFiberComposites.pdf (accessed on 22 February 2014).
- Han, G.; Stokke, D.D.; Wu, Q.; Han, G. Introduction to Wood and Natural Fiber Composites; John Wiley and Sons: New York, NY, USA, 2014. [Google Scholar]
- Dodd, R.B.; Akin, D.E. Recent Developments in Retting and Measurement of Fiber Quality. In Natural Fibers, Biopolymers, and Biocomposites; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; Taylor & Francis Group: New York, NY, USA, 2005. [Google Scholar]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical treatments of natural fiber for use in natural fiber-reinforced composites: A review. Sci. Bus. Media 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Miller, D.J.; McMullin, D.R.; Sumarah, M.W. Chaetoglobosins and azaphilones produced by Canadian strains of Chaetomium globosum isolated from the indoor environment. Mycotoxin Res. 2012, 29, 47–54. [Google Scholar]
- Pekhtasheva, E.; Neverov, A.; Kubica, S.; Zaikov, G. Biodegradation and biodeterioration of some natural polymers. Chem. Chem. Technol. 2012, 6, 263–280. [Google Scholar]
- Kozlowski, R.; Walentowska, J. Role of biocides from plant origin in protection of natural fibers against biodeterioration. In Proceedings of the International Conference on Flax and Other Bast Plants, Saskatoon, SK, Canada, 21–23 July 2009; pp. 326–331. [Google Scholar]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibers as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Biles, C.L.; Cluck, T.; Fuego, M.; Guinn, A.; Martin, M.; Poudayl, S.; Wright, D.; Young, J. Differential chlorate inhibition of Chaetomium globosum Germination, hyphal growth, and perithecia synthesis. Mycopathologia 2012, 174, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.; Khalil, A.H.P.S. Cellulosic/synthetic fiber reinforced polymer hybrid composites: A review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Nielsen, P.A.; Thrane, U.; Larsen, T.O.; Gravesen, S. Production of mycotoxins on artificially inoculated building materials. Int. Biodeterior. Biodegrad. 1998, 42, 9–16. [Google Scholar] [CrossRef]
- Liang, J.Z. Predictions of young’s modulus of short inorganic fiber reinforced polymer composites. Compos. Part B Eng. 2012, 43, 1763–1766. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Sukumaran, K.; Mukherjee, P.S.; Pavithran, C.; Pillai, S.G.K. Natural fiber-polymer composites. Cem. Concr. Compos. 1990, 12, 117–136. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Mechanical property evaluation of sisal-jute glass fiber reinforced polyester composites. Compos. Part B Eng. 2013, 48, 1–9. [Google Scholar] [CrossRef]
- Dobreva, D.; Nenkova, S.; Vasileva, St. Morphology and mechanical properties of polypropylene-wood flour composites. BioResources 2006, 1, 209–219. [Google Scholar]
- Lopez, J.P.; Mutje, P.; Pelach, M.A.; Mansouri, M.E.E.; Boufi, S.; Vilaseca, F. Analysis of the tensile modulus of polypropylene composites reinforced with stone groundwood fibers. BioResources 2012, 7, 1310–1323. [Google Scholar] [CrossRef]
- Bourmaud, A.; Morvan, C.; Baley, C. Importance of fiber preparation to optimize the surface and mechanical properties of unitary flax fiber. Ind. Crop. Prod. 2010, 32, 662–667. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H.; Alam, A.K.M.M.; Beg, M.D.H.; Khan, M.A.; Gafur, M.A. Mechanical and degradation characteristics of natural silk and synthetic phosphate glass fiber reinforced polyp composites. J. Compos. Mater. 2010, 45, 1305–1313. [Google Scholar] [CrossRef]
- Venkateshwaran, N.; Elayaperumal, A. Banana fiber reinforced polymer composites-a review. J. Reinf. Plast. Compos. 2010, 29, 2387–2396. [Google Scholar] [CrossRef]
- Tajvidi, M.; Takemura, A. Thermal degradation of natural fiber-reinforced polypropylene composites. Thermoplast. Compos. Mater. 2009, 23, 281–298. [Google Scholar] [CrossRef]
- Anne, P.; Anne, L. Should I Choose a Hemp or Linen Fabric? Available online: https://oecotextiles.wordpress.com/2015/08/05/should-i-choose-a-hemp-or-linen-fabric/ (accessed on 28 October 2017).
- Georgiopoulos, P.; Christopoulos, A.; Koutsoumpis, S.; Kontou, E. The effect of surface treatment on the performance of flax/biodegradable composites. Compos. Part B Eng. 2016, 106, 88–98. [Google Scholar] [CrossRef]







| Chemical Composition & Geometrical Features | Mat Type | |||
|---|---|---|---|---|
| H-1381 (Hemp-PP) | F-1375 (Flax-PP) | H-1416 (Hemp) | F-1401 (Flax) | |
| Cellulose (%) | 67.2 | 69.8 | 72.8 | 73.8 |
| Hemicellulose (%) | 15.7 | 14.2 | 14.0 | 13.0 |
| Lignin (%) | 13.5 | 11.7 | 10.3 | 10.3 |
| Shive (%) | 5.8 | 17.1 | 10.8 | 10.4 |
| Fiber diameter (μm) | 39.4 | 22.5 | 32.1 | 29.9 |
| Fiber length (mm) | 5–10 | 2–5 | 2–5 | 10–15 |
| Fiber surface area (μm2) | 928.3 × 103 | 247.4 × 103 | 353.0 × 103 | 1174.2 × 103 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, B.; Pakpour, S.; Kazemian, N.; Klironomos, J.; Stoeffler, K.; Rho, D.; Denault, J.; Milani, A.S. Effect of Fungal Deterioration on Physical and Mechanical Properties of Hemp and Flax Natural Fiber Composites. Materials 2017, 10, 1252. https://doi.org/10.3390/ma10111252
Crawford B, Pakpour S, Kazemian N, Klironomos J, Stoeffler K, Rho D, Denault J, Milani AS. Effect of Fungal Deterioration on Physical and Mechanical Properties of Hemp and Flax Natural Fiber Composites. Materials. 2017; 10(11):1252. https://doi.org/10.3390/ma10111252
Chicago/Turabian StyleCrawford, Bryn, Sepideh Pakpour, Negin Kazemian, John Klironomos, Karen Stoeffler, Denis Rho, Johanne Denault, and Abbas S. Milani. 2017. "Effect of Fungal Deterioration on Physical and Mechanical Properties of Hemp and Flax Natural Fiber Composites" Materials 10, no. 11: 1252. https://doi.org/10.3390/ma10111252
APA StyleCrawford, B., Pakpour, S., Kazemian, N., Klironomos, J., Stoeffler, K., Rho, D., Denault, J., & Milani, A. S. (2017). Effect of Fungal Deterioration on Physical and Mechanical Properties of Hemp and Flax Natural Fiber Composites. Materials, 10(11), 1252. https://doi.org/10.3390/ma10111252