Biocompatibility and Corrosion Protection Behaviour of Hydroxyapatite Sol-Gel-Derived Coatings on Ti6Al4V Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Hydroxyapatite Sol
2.2. Preparation of HAp Coatings on Ti6Al4V Substrates
2.3. Characterization of HAp Powder Samples
2.3.1. Thermal Behaviour (DTA/TGA)
2.3.2. Fourier Transforms Infrared Spectrometer (FTIR)
2.3.3. X-ray Diffraction Analysis (XRD)
2.4. Characterization of the HAp Sol-Gel Coatings
2.4.1. Coating Thickness
2.4.2. Adhesion Measurements
2.4.3. Roughness Measurements
2.5. Evaluation of the Bioactivity
2.5.1. Immersion Tests of HAp-Coatings/Ti6Al4V System in SBF
2.5.2. Inductively Coupled Plasma Technique (ICP)
2.5.3. Scanning Electron Microscopy (SEM)
2.6. Biocompatibility Studies
2.6.1. Tests with Cell Line of Human Fetal Osteoblasts
2.6.2. Cytotoxicity of Leachates. MTT Assay
2.6.3. Analysis of Cell Adhesion and Proliferation on the Surface of the Materials. Alamar Blue™ Assay
2.6.4. Analysis by SEM of the Cultures Established on the Materials Surface
2.7. Corrosion Protection Behaviour
3. Results and Discussion
3.1. Characterization of HAp Powder Samples
- I300: Intensity of peak diffracted from the (300) crystallographic planes of HAp.
- V112/300: Intensity of the valley between the peaks of the planes (112) and (300).
- Lc: Average crystallite size (nm).
- K: Shape coefficient (value between 0.9 and 1.0).
- λ: Wavelength of X-ray beam-Cu Kα radiation (λ = 0.15406 nm).
- β: Full width at half maximum (FWHM) of HAp(211).
- θ: Diffraction angle.
3.2. Characterization of the HAp Sol-Gel Coatings
3.3. Bioactivity Assessments of the HAp-Coatings/Ti6Al4V System
3.4. Biocompatibility Evaluation of Sol-Gel-Derived HAp Coatings Deposited on Ti6Al4V Surfaces. Cell Viability and Proliferation
3.4.1. Cell Viability-Absorbance Measurements
3.4.2. Cell Proliferation-Alamar Blue™ Assay
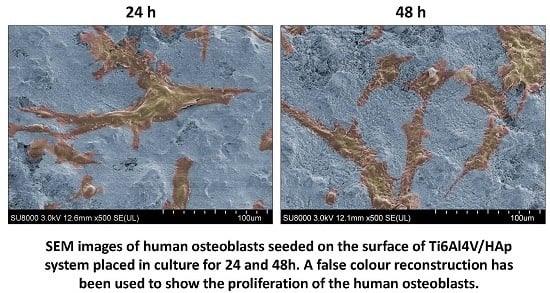
3.4.3. Inspection of the Cultures Established on the Materials Surface-SEM
3.5. Corrosion Behaviour
3.5.1. Selection of the Electrical Equivalent Circuit
3.5.2. Interpretation of the Impedance Spectra
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vallet-Regi, M.; Gonzalez-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Barranco, V.; Jiménez-Morales, A.; Peón, E.; Galván, J.C. Multifunctional sol-gelderived thin film based on nanocrystaline hydroxyapatite powders. J. Phys. Conf. Ser. 2010, 252, 012007. [Google Scholar] [CrossRef]
- Henc, L.L. Bioceramic. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Ayatollahi, M.R.; Beigzadeh, B. Preparation and characterisation of hydroxyapatite derived from natural bovine bone and PMMA/BHA composite for biomedical applications. Mater. Technol. 2016, 31, 448–453. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, J.; Yang, J.; Wang, Y.; Zhang, C.; Ke, Q. Hybrid nanostructured hydroxyapatite–chitosan composite scaffold: Bioinspired fabrication, mechanical properties and biological properties. J. Mater. Chem. B 2015, 3, 4679–4689. [Google Scholar] [CrossRef]
- Wu, H.-C.; Wang, T.-W.; Sun, J.-S.; Lee, Y.-H.; Shen, M.-H.; Tai, Z.-R.; Chen, C.-Y.; Hsu, H.-C. Development and characterization of a bioinspired bone matrix with aligned nanocrystalline hydroxyapatite on collagen nanofibers. Materials 2016, 9, 198. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review studyon the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.D.A.; Sun, Z.F.; Ali, A. A review of synthesis methods, properties and use of hydroxyapatite as a substitute of bone. J. Biomim. Biomater. Biomed. Eng. 2015, 25, 98–117. [Google Scholar] [CrossRef]
- Liu, D.M.; Oczynski, T.T.; Tseng, W.J. Water-based sol-gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Hsieh, M.F.; Perng, L.H.; Chin, T.S.; Perng, H.G. Phase purity of sol–gel-derived hydroxyapatite ceramic. Biomaterials 2001, 22, 2601–2607. [Google Scholar] [CrossRef]
- Avés, E.P.; Estévez, G.F.; Sader, M.S.; Sierra, J.C.G.; Yurell, J.C.L.; Bastos, I.N.; Soares, G.D.A. Hydroxyapatite coating by sol–gel on Ti–6Al–4V alloy as drug carrier. J. Mater. Sci. Mater. Med. 2009, 20, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Peón, E.; El-Hadad, A.A.; García-Galván, F.R.; Jiménez-Morales, A.; Galván, J.C. Controlled Rate Thermal Analysis (CRTA) as new method to control the specific surface of Hydroxyapatite thin coatings. In Modern Technologies for Creating the Thin-film Systems and Coatings; Nikitenkov, N., Ed.; InTech: Rijeka, Croatia, 2017; accepted, in press. [Google Scholar]
- De Groot, K.; Klein, C.P.A.T.; Wolke, J.G.C.; de Blieck-Hogervorst, J.M.A. CRC Handbook of Bioactive Calcium Phosphates; Yamamuro, T., Hench, L.L., Wilson, J., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume 2, pp. 3–16. [Google Scholar]
- Doorn, P.F.; Campbell, P.A.; Worrall, J.; Benya, P.D.; Amstutz, H.C. Metal wear particle characterization from metal on metal total hip replacements: Transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 1998, 42, 103–111. [Google Scholar] [CrossRef]
- Leidheiser, H., Jr. Electrochemical methods for appraising corrosion protective coatings. J. Coat. Technol. 1991, 63, 21–31. [Google Scholar]
- Galván, J.C.; Larrea, M.T.; Braceras, I.; Multigner, M.; González-Carrasco, J.L. In vitro corrosion behaviour of surgical 316LVM stainless steel modified by Si+ ion implantation–An electrochemical impedance spectroscopy study. J. Alloys Compd. 2016, 676, 414–427. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Revisiting ceramics for medical applications. Dalton Trans. 2006, 44, 5211–5220. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Ordered mesoporous materials in the context of drug delivery systems and bone tissue engineering. Chem. A Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Govers, R.; James, D.E.; Coster, A.C.F. High-throughput analysis of the dynamics of recycling cell surface proteins. In Exocytosis and Endocytosis, Methods in Molecular Biology Series; Andrei, I.I., Ed.; Springer Science Business Media: New York, NY, USA, 2008; Volume 440, pp. 129–146. [Google Scholar]
- Wan, H.; Williams, R.; Doherty, P.; Williams, D.F. A study of the reproducibility of the MTT test. J. Mater. Sci. Mater. Med. 1994, 5, 154–159. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Shubert, D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Gloeckner, H.; Jonuleit, T.; Lemke, H.D. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue. J. Immunol. Methods 2001, 252, 131–138. [Google Scholar] [CrossRef]
- Wang, D.G.; Chen, C.Z.; Ting, H.; Lei, T. Hydroxyapatite coating on Ti6Al4V alloy by a sol-gel method. J. Mater. Sci. Mater. Med. 2008, 19, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kumta, P.N. Sol-gel synthesis and characterization of nanostructured Hydroxyapatite powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Wang, D.G.; Chen, C.Z.; Ma, J.; Zhang, G. In situ synthesis of hydroxyapatite coating by laser cladding. Colloid Surf. B Biointerfaces 2008, 66, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhang, S.; Weng, W.J. Sol-gel Preparation of Fluoridated Hydroxyapatite in Ca(NO3)2-PO(OH)3−x(OEt)x-HPF6 System. J. Sol-Gel Sci. Technol. 2006, 38, 13–17. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Hamdi, M.; Ramesh, S. Properties of hydroxyapatite produced by annealing of bovine bone. Ceram. Int. 2007, 33, 1171–1177. [Google Scholar] [CrossRef]
- Rajabi-Zamani, A.H.; Behnamghader, A.; Kazemzadeh, A. Synthesis of nanocrystalline carbonated hydroxyapatite powder via nonalkoxide sol-gel method. Mater. Sci. Eng. C 2008, 28, 1326–1329. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Klung, H.P.; Alexander, E. X-ray Diffraction Procedures: For Polycrystallite and Amorphous Materials, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- El Hadad, A.A. An Approach to Design New Coatings for Biomedical Applications. Ph.D. Thesis, Universidad Carlos III de Madrid, Leganés (Madrid), Spain, October 2012. [Google Scholar]
- Kokubo, C.O.T.; Yamamuro, T. Mechanism of apatite formation on CaO-SiO2-P2O5 glasses in a simulated body fluid. J. Non Cryst. Solids 1992, 143, 84–92. [Google Scholar]
- Kim, H.M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The mechanism of biomineralization of bone-like apatite on synthetic hydroxyapatite: An in vitro assessment. J. R. Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Macdonald, J.R. Impedance spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- El Hadad, A.A.; Barranco, V.; Jiménez-Morales, A.; Hickman, G.J.; Galván, J.C.; Perry, C.C. Triethylphosphite as a network forming agent enhances in-vitro biocompatibility and corrosion protection of hybrid organic-inorganic sol-gel coatings for Ti6Al4V alloys. J. Mater. Chem. B 2014, 2, 7955–7963. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Barranco, V.; Jiménez-Morales, A.; Peón, E.; Hickman, G.J.; Perry, C.C.; Galván, J.C. Enhancing in vitro biocompatibility and corrosion protection of organic-inorganic hybrid sol-gel films with nanocrystalline hydroxyapatite. J. Mater. Chem. B 2014, 2, 3886–3896. [Google Scholar] [CrossRef]
- Walter, G.W. A review of impedance plot methods used for corrosion performance analysis of painted metals. Corr. Sci. 1986, 26, 681–703. [Google Scholar] [CrossRef]
- Feliu, S.; Galván, J.C.; Morcillo, M. An interpretation of electrical impedance diagrams for painted galvanized steel. Prog. Org. Coat. 1989, 17, 143–153. [Google Scholar] [CrossRef]
- Chico, B.; Galván, J.C.; de la Fuente, D.; Morcillo, M. Electrochemical impedance spectroscopy study of the effect of curing time on the early barrier properties of silane systems applied on steel substrates. Prog. Org. Coat. 2007, 60, 45–53. [Google Scholar] [CrossRef]
- Barranco, V.; Carmona, N.; Galván, J.C.; Grobelny, M.; Kwiatkowski, L.; Villegas, M.A. Electrochemical study of tailored sol-gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog. Org. Coat. 2010, 68, 347–355. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Silverman, D.C. Technical Note: On Ambiguities in Modeling Electrochemical ImpedanceSpectra Using Circuit Analogues. Corrosion 1991, 47, 87–89. [Google Scholar] [CrossRef]


















| Sample | HAp Sol-Gel-Derived Powders | ||
|---|---|---|---|
| Thermal Treatment T (°C) | Crystallite Size (nm) | Degree of Crystallinity (%) | |
| S6 | 600 | 40 | 85 |
| S8 | 800 | 51 | 89 |
| S12 | 1200 | 54 | 82 |
| Sample | HAp Sol-Gel-Derived Coatings | |
|---|---|---|
| Thermal Treatment T (°C) | Thickness (µm) | |
| S6 | 600 | 7.74 ± 0.655 |
| S8 | 800 | 8.51 ± 0.135 |
| S12 | 1200 | 8.63 ± 0.121 |
| Frequency | Mean | Median | Geometric Mean | Variance | Standard Deviation | Standard Error | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| 5 | 29.036 | 28.94 | 28.8462 | 13.1609 | 3.6278 | 1.6224 | 23.54 | 33.35 |
| Sample | Ra (µm) | Rp (µm) | Rv (µm) |
|---|---|---|---|
| Ti6Al4V (nude) | 0.094 ± 0.018 | 0.467 ± 0.153 | −0.361 ± 0.104 |
| S6 (TT 600 °C/2 h) | 0.163 ± 0.028 | 1.390 ± 0.911 | −0.650 ± 0.155 |
| S8 (TT 800 °C/2 h) | 0.165 ± 0.012 | 0.631 ± 0.150 | −0.606 ± 0.103 |
| Electrical Element | Equivalent Circuit A | Equivalent Circuit B | ||||
|---|---|---|---|---|---|---|
| Value | Error | Error % | Value | Error | Error % | |
| Rs | 138.8 | 3.43 | 2.47 | 126.6 | 2.95 | 2.33 |
| Rcoat | 1014.0 | 49.36 | 4.93 | 747.3 | 37.51 | 5.02 |
| CPEcoat-Y0 | 2.32 × 10−6 | 3.46 × 10−7 | 14.93 | 3.36 × 10−6 | 5.30 × 10−7 | 15.8 |
| CPEcoat-n | 0.67 | 0.01 | 2.17 | 0.68 | 0.02 | 2.56 |
| CPEdl-Y0 | 7.05 × 10−5 | 5.99 × 10−7 | 0.85 | 7.16 × 10−5 | 5.69 × 10−7 | 0.79 |
| CPEdl-n | 0.46 | 4.00 × 10−3 | 0.86 | 0.46 | 4.59 × 10−3 | 0.99 |
| Rcorr | 1.46 × 105 | 6.99 × 103 | 4.77 | 1.51 × 105 | 7.96 × 103 | 5.27 |
| Chi-Squared (χ2) | 0.0009 | 0.0012 | ||||
| Immersion Time in SBF | CPEdl-n Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample S6 (TT: 600 °C/2 h) | Sample S8 TT: 800 °C/2 h | |||||||
| Equivalent Circuit A | Equivalent Circuit B | Equivalent Circuit A | Equivalent Circuit B | |||||
| Value | Error % | Value | Error % | Value | Error % | Value | Error % | |
| 5 min | 0.525 | 0.96 | 0.613 | 5.61 | 0.455 | 1.97 | 0.476 | 3.11 |
| 1 day | 9.268 | 9.35 | 0.628 | 1.18 | 0.415 | 0.80 | 0.410 | 1.22 |
| 2 days | 1.933 | 8.31·103 | 0.624 | 21.9 | 0.422 | 0.77 | 0.423 | 1.02 |
| 5 days | 0.651 | 5.99 | 0.644 | 3.43 | 0.464 | 0.86 | 0.463 | 0.99 |
| 9 days | 0.491 | 2.96 | 0.513 | 1.47 | 0.477 | 0.92 | 0.478 | 1.05 |
| 15 days | 0.630 | 1.20 | 0.636 | 0.98 | 0.511 | 1.03 | 0.516 | 1.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hadad, A.A.; Peón, E.; García-Galván, F.R.; Barranco, V.; Parra, J.; Jiménez-Morales, A.; Galván, J.C. Biocompatibility and Corrosion Protection Behaviour of Hydroxyapatite Sol-Gel-Derived Coatings on Ti6Al4V Alloy. Materials 2017, 10, 94. https://doi.org/10.3390/ma10020094
El Hadad AA, Peón E, García-Galván FR, Barranco V, Parra J, Jiménez-Morales A, Galván JC. Biocompatibility and Corrosion Protection Behaviour of Hydroxyapatite Sol-Gel-Derived Coatings on Ti6Al4V Alloy. Materials. 2017; 10(2):94. https://doi.org/10.3390/ma10020094
Chicago/Turabian StyleEl Hadad, Amir A., Eduardo Peón, Federico R. García-Galván, Violeta Barranco, Juan Parra, Antonia Jiménez-Morales, and Juan Carlos Galván. 2017. "Biocompatibility and Corrosion Protection Behaviour of Hydroxyapatite Sol-Gel-Derived Coatings on Ti6Al4V Alloy" Materials 10, no. 2: 94. https://doi.org/10.3390/ma10020094
APA StyleEl Hadad, A. A., Peón, E., García-Galván, F. R., Barranco, V., Parra, J., Jiménez-Morales, A., & Galván, J. C. (2017). Biocompatibility and Corrosion Protection Behaviour of Hydroxyapatite Sol-Gel-Derived Coatings on Ti6Al4V Alloy. Materials, 10(2), 94. https://doi.org/10.3390/ma10020094