Silk: Optical Properties over 12.6 Octaves THz-IR-Visible-UV Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spectroscopy Setups and Techniques
2.2. Silk Samples
3. Results and Discussion
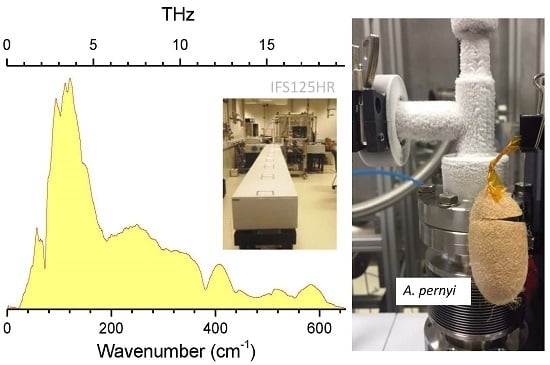
3.1. T-Rays
3.2. Mid-IR and IR Range
3.3. Vis-UV Range
3.4. Discussion of the Band Assignment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mlnonl, G.; Zerbl, G. End Effects on Longitudinal Accordion Modes: Fatty Acids and Layered Systems. J. Phys. Chem. 1982, 86, 4791–4798. [Google Scholar]
- Mizushima, S.; Shimanouchi, T. Raman frequencies of n-paraffin molecules. J. Am. Chem. Soc. 1949, 7, 1320–1324. [Google Scholar] [CrossRef]
- Doucet, J.; Denicolo, I.; Craievich, A. X-ray study of the rotator phase of the odd numbered paraffins C17H36, C19H40, and C21H44. J. Chem. Phys. 1981, 75, 1523. [Google Scholar] [CrossRef]
- Barnes, J.D.; Fanconi, B.M. Raman Spectroscopy, Rotational Isomerism, and the “Rotator” Phase Transition in n—Alkanes. J. Chem. Phys. 1972, 56, 5190–5192. [Google Scholar] [CrossRef]
- Chumakov, A.I.; Monaco, G.; Monaco, A.; Crichton, W.A.; Bosak, A.; Rüffer, R.; Meyer, A.; Kargl, F.; Comez, L.; Fioretto, D.; et al. Equivalence of the Boson Peak in Glasses to the Transverse Acoustic van Hove Singularity in Crystals. Phys. Rev. Lett. 2011, 106, 225501. [Google Scholar] [CrossRef] [PubMed]
- Png, G.M.; Falconer, R.J.; Abbott, D. Tracking Aggregation and Fibrillation of Globular Proteins Using Terahertz and Far-Infrared Spectroscopies. IEEE Trans. Terahertz Ssci. Technol. 2016, 6, 45. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.Q. Silk Fiber—Molecular Formation Mechanism, Structure- Property Relationship and Advanced Applications. In Oligomerization of Chemical and Biological Compounds; Lesieur, C., Ed.; Intech: Rijeka, Croatia, 2014. [Google Scholar]
- Kujala, S.; Mannila, A.; Karvonen, L.; Kieu, K.; Sun, Z. Natural Silk as a Photonics Component: A Study on Its Light Guiding and Nonlinear Optical Properties. Sci. Rep. 2016, 6, 22358. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk Materials: A Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Cher, G.; He, J.; Han, Y.; Wang, X.; Kaplan, D.L. Silk-Based Biomaterials in Biomedical Textiles and Fiber-Based Implants. Adv. Healthc. Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marelli, B.; Brenckle, M.A.; Mitropoulos, A.N.; Gil, E.S.; Tsioris, K.; Tao, H.; Kaplan, D.L.; Omenetto, F.G. All-water-based electron-beam lithography using silk as a resist. Nat. Nanotechnol. 2014, 9, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Balčytis, A.; Ryu, M.; Seniutinas, G.; Juodkazytė, J.; Cowie, B.C.C.; Stoddart, P.R.; Morikawa, J.; Juodkazis, S. Black-CuO: Surface-enhanced Raman scattering and infrared properties. Nanoscale 2015, 7, 18299–18304. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Li, Q.; Sun, S.M.; Huang, J.C.; Zheng, B.Y.; Chen, Q.D.; Shao, Z.Z.; Sun, H.B. Aqueous multiphoton lithography with multifunctional silk-centred bio-resists. Nat. Commun. 2015, 6, 8612. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Delivery Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.S.; Park, S.H.; Cebe, P.; Kaplan, D.L. Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, H.; Leow, W.R.; Cai, Y.; Loh, X.J.; Han, M.Y.; Chen, X. Silk Fibroin for Flexible Electronic Devices. Adv. Mater. 2016, 28, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Oua, J.Z.; Daeneke, T.; Mitchell, A.; Sasaki, T.; Fuhrer, M.S. Two dimensional and layered transition metal oxides. Appl. Mater. Today 2016, 5, 73–89. [Google Scholar] [CrossRef]
- Fei, X.; Shao, Z.; Chen, X. Synthesis of hierarchical three-dimensional copper oxide nanostructures through a biomineralization-inspired approach. Nanoscale 2013, 5, 7991–7997. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Zhu, G.; Kaplan, D.L.; Cao, C.; Zhu, H.; Lu, Q. Silk-regulated hierarchical hollow magnetite/carbon nanocomposite spheroids for lithium-ion battery anodes. Nanotechnology 2015, 26, 115603. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, X.; Lu, Q.; Sheng, W.; Liu, L.; Dong, B.; Kaplan, D.L.; Zhu, H. Reversible Hydrogel–Solution System of Silk with High Beta-Sheet Content. Nanotechnology 2014, 15, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Cebe, P.; Hu, X.; Kaplan, D.L.; Zhuravlev, E.; Wurm, A.; Arbeiter, D.; Schick, C. Beating the Heat—Fast Scanning Melts Silk Beta Sheet Crystals. Sci. Rep. 2013, 3, 1130. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, J.; Ryu, M.; Balčytis, A.; Seniutinas, G.; Fan, L.; Mizeikis, V.; Li, J.L.; Wang, X.W.; Zamengo, M.; Wang, X.; et al. Silk fibroin as water-soluble bio-resist and its thermal properties. RSC Adv. 2015, 6, 11863–11869. [Google Scholar] [CrossRef]
- Hebling, J.; Yeh, K.L.; Hoffmann, M.C.; Bartal, B.; Nelson, K.A. Generation of high-power terahertz pulses by tilted-pulse-front excitation and their application possibilities. J. Opt. Soc. Am. B 2008, 25, B6–B19. [Google Scholar] [CrossRef]
- Novelli, F.; Chon, J.W.M.; Davis, J.A. Terahertz thermometry of gold nanospheres in water. Opt. Lett. 2016, 41, 5801–5804. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Sharma, G.; Razzari, L.; Ropagnol, X.; Bandulet, H.C.; Vidal, F.; Morandotti, R.; Kieffer, J.C.; Ozaki, T.; Tiedje, H.; et al. Generation of Intense Terahertz Radiation via Optical Methods. IEEE J. Sel. Top. Quant. Electron. 2011, 17, 5–16. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Matsuo, S.; Ueno, K.; Misawa, H. Three-Dimensional Micro- and Nano-Structuring of Materials by Tightly Focused Laser Radiation. Bull. Chem. Soc. Jpn. 2008, 81, 411–448. [Google Scholar] [CrossRef]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2010, 10, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sezutsu, H.; Yukuhiro, K. Dynamic Rearrangement Within the Antheraea pernyi Silk Fibroin Gene Is Associated with Four Types of Repetitive Units. J. Mol. Evol. 2000, 51, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Shao, H.; Hu, X. Antheraea pernyi Silk Fiber: A Potential Resource for Artificially Biospinning Spider Dragline Silk. J. Biomed. Biotechnol. 2010, 2010, 683962. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Leigen, L.; Zhengyu, W. Effect on Properties of Regenerated Silk Fibroin Fiber Coagulated with Aqueous Methanol/Ethanol. J. Appl. Polym. Sci. 2007, 106, 53–59. [Google Scholar] [CrossRef]
- Ling, S.; Qi, Z.; Knight, D.P.; Huang, Y.; Huang, L.; Zhou, H.; Shao, Z.; Chen, X. Insight into the structure of single Antheraea pernyi silkworm fibers using synchrotron FTIR microspectroscopy. Biomacromolecules 2013, 14, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.J.; Markelz, A.G. Terahertz spectroscopic analysis of peptides and proteins. J. Infrared Milli. Terahz. Waves 2012, 33, 973–988. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yun, Y.S.; Lee, S.; Jang, D.; Park, K.Y.; Kim, J.K.; Kim, B.H.; Kang, K.; Kaplan, D.L.; Jin, H.J. Carbonization of a stable β-sheet-rich silk protein into a pseudographitic pyroprotein. Nat. Commun. 2015, 6, 7145. [Google Scholar] [CrossRef] [PubMed]
- Zelsmann, H.R. Temperature dependence of the optical constants for liquid H2O and D2O in the far IR region. J. Mol. Struct. 1995, 350, 95–114. [Google Scholar] [CrossRef]
- Brubach, J.B.; Mermet, A.; Filabozzi, A.; Gerschel, A.; Roy, P. Signatures of the hydrogen bonding in the infrared bands of water. J. Chem. Phys. 2005, 122, 184509. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of Water Content on the β-Sheet Formation, Thermal Stability, Water Removal, and Mechanical Properties of Silk Materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Medcraft, C.; McNaughton, D.; Thompson, C.D.; Appadoo, D.; Bauerecker, S.; Robertson, E.G. Size and temperature dependence in the far-IR spectra of water ice particles. Astrophys. J. 2012, 758, 17. [Google Scholar] [CrossRef]
- Sun, C.Q.; Zhang, X.; Fu, X.; Zheng, W.; Kuo, J.L.; Zhou, Y.; Shen, Z.; Zhou, J. Density and Phonon-Stiffness Anomalies of Water and Ice in the Full Temperature Range. J. Phys. Chem. Lett. 2013, 4, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Nakahara, T.; Shimanouchi, T.; Oya, M.; Uno, K.; Iwakura, Y. Far-infrared spectra of polyalanines with α-helical and β-form structures. Biopolymers 1968, 6, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.J.; Zakaria, H.A.; Fan, Y.Y.; Bradley, A.P.; Middelberg, A.P. Far-Infrared Spectroscopy of Protein Higher-Order Structures. Appl. Spectrosc. 2010, 64, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Sölter, J.; Kremer, F. Structure-property relationships in major ampullate spider silk as deduced from polarized FTIR spectroscopy. Eur. Phys. J. E 2007, 24, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.; Monti, P. Vibrational infrared conformational studies of model peptides representing the semicrystalline domains of Bombyx mori silk fibroin. Biopolymers 2005, 78, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Taddei, P.; Freddi, G.; Ohgo, K.; Asakura, T. Vibrational 13C-Cross-Polarization/Magic Angle Spinning NMR Spectroscopic and Thermal Characterization of Poly(alanine-glycine) as Model for Silk I Bombyx mori Fibroin. Biopolymers 2003, 72, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.H.; Krimm, S. Vibrational analysis of peptides, polypeptides, and proteins. II. β-poly(l-alanine) and β-poly(l-alanylglycine). Biopolymers 1976, 15, 2465–2483. [Google Scholar]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wu, P.; Chen, X.; Shao, Z. Near-Infrared Characterization on the Secondary Structure of Regenerated Bombyx Mori Silk Fibroin. Appl. Spectrosc. 2006, 60, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yao, J.; Huang, L.; Chen, X.; Shao, Z. Correlation between structural and dynamic mechanical transitions of regenerated silk fibroin. Polymer 2010, 51, 6278–6283. [Google Scholar] [CrossRef]
- Millington, K.R. Diffuse reflectance spectroscopy of fibrous proteins. Amino Acids 2012, 43, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Rajkhowa, R.; Tsuzuki, T.; Millington, K.; Zhang, J.; Wang, X. Photoprotection by Silk Cocoons. Biomacromolecules 2013, 14, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, A.; Goutham Vishnu, B.; Thippa Reddy, K.S. Preparation of nano silk sericin based hydrogels from silk industry waste. J. Environ. Res. Develop. 2013, 8, 243–253. [Google Scholar]
- Noble, J.E.; Bailey, M.J.A. Chapter 8 Quantitation of Protein. In Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 73–95. [Google Scholar]
- Ding, T.; Huber, T.; Middelberg, A.P.; Falconer, R.J. Characterization of Low-Frequency Modes in Aqueous Peptides Using Far-Infrared Spectroscopy and Molecular Dynamics Simulation. J. Phys. Chem. A 2011, 115, 11559–11565. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, T.; Garrido, C.; Clavijo, R.E.; Gómez-Jeria, J.S.; Monasterio, C.A.; Icaza, M.; Moraga, F.E.; Vallette, M.M.C. Raman and surface enhanced Raman scattering of a black dyed silk. J. Raman Spectrosc. 2013, 44, 1238–1245. [Google Scholar] [CrossRef]
- Walrafen, G.E. Raman Spectrum of Water: Transverse and Longitudinal Acoustic Modes below 300 cm−1 and Optic Modes above 300 cm−1. J. Phys. Chem. 1990, 94, 2237–2239. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Maximova, K.; Wang, X.W.; Balčytis, A.; Fan, L.; Li, J.; Juodkazis, S. Silk patterns made by direct femtosecond laser writing. Biomicrofluidics 2016, 10, 054101. [Google Scholar] [CrossRef] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčytis, A.; Ryu, M.; Wang, X.; Novelli, F.; Seniutinas, G.; Du, S.; Wang, X.; Li, J.; Davis, J.; Appadoo, D.; et al. Silk: Optical Properties over 12.6 Octaves THz-IR-Visible-UV Range. Materials 2017, 10, 356. https://doi.org/10.3390/ma10040356
Balčytis A, Ryu M, Wang X, Novelli F, Seniutinas G, Du S, Wang X, Li J, Davis J, Appadoo D, et al. Silk: Optical Properties over 12.6 Octaves THz-IR-Visible-UV Range. Materials. 2017; 10(4):356. https://doi.org/10.3390/ma10040356
Chicago/Turabian StyleBalčytis, Armandas, Meguya Ryu, Xuewen Wang, Fabio Novelli, Gediminas Seniutinas, Shan Du, Xungai Wang, Jingliang Li, Jeffrey Davis, Dominique Appadoo, and et al. 2017. "Silk: Optical Properties over 12.6 Octaves THz-IR-Visible-UV Range" Materials 10, no. 4: 356. https://doi.org/10.3390/ma10040356
APA StyleBalčytis, A., Ryu, M., Wang, X., Novelli, F., Seniutinas, G., Du, S., Wang, X., Li, J., Davis, J., Appadoo, D., Morikawa, J., & Juodkazis, S. (2017). Silk: Optical Properties over 12.6 Octaves THz-IR-Visible-UV Range. Materials, 10(4), 356. https://doi.org/10.3390/ma10040356