Improving Sorbents for Glycerol Capture in Biodiesel Refinement
Abstract
:1. Introduction
2. Results
2.1. Sorbents
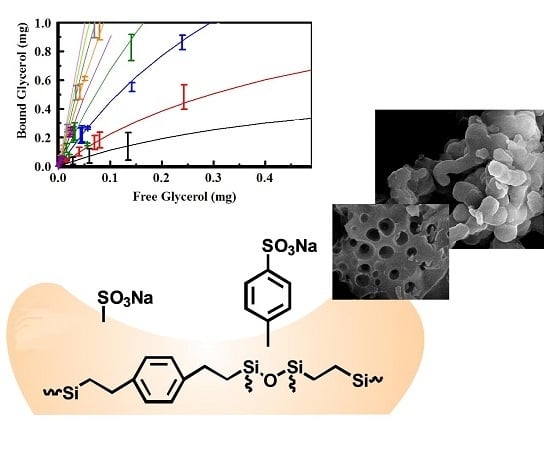
2.2. Glycerol Capture from Aqueous Solution
2.3. Glycerol Capture from Biodiesel
3. Materials and Methods
3.1. Synthesis of E25-Ph(2) and E50-Ph(2) (Type 2, Ethane-Bridged Organosilicates, Ph Indicates Grafted Phenyl Groups)
3.2. Synthesis of ED11-Ph1(3), ED11-Ph2(3), and ED11a(4) (Types 3 and 4, Mixed Ethane and Diethylbenzene-Bridged Organosilicates, Ph Indicates Grafted Phenyl Groups)
3.3. Synthesis of S65-Ph(1) and S85-Ph(1) (Type 1, silicates, Ph Indicates Grafted Phenyl Groups)
3.4. Synthesis of D13a(6), D13b(6), D13c(6), D13-Ph1(5), and D13-Ph2(5) (Types 5 and 6, Diethylbenzene-Bridged Organosilicates, Ph Indicates Grafted Phenyl Groups)
3.5. Synthesis of PDVB-2 and PDVB-3 (Poly(Divinylbenzene) Resins)
3.6. Characterization
3.7. Biodiesel Synthesis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bender, M. Economic feasibility review for community-scale farmer cooperatives for biodiesel. Bioresour. Technol. 1999, 70, 81–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- You, Y.D.; Shie, J.L.; Chang, C.Y.; Huang, S.H.; Pai, C.Y.; Yu, Y.H.; Chang, C.F.H. Economic cost analysis of biodiesel production: Case in soybean oil. Energy Fuels 2008, 22, 182–189. [Google Scholar] [CrossRef]
- Araujo, V.; Hamacher, S.; Scavarda, L.F. Economic assessment of biodiesel production from waste frying oils. Bioresour. Technol. 2010, 101, 4415–4422. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil-an economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Faccini, C.; da Cunha, M.; Moraes, M.; Krause, L.; Manique, M.; Rodrigues, M.; Benvenutti, E.; Caramao, E. Dry washing in biodiesel purification: A Comparitive study of adsorbents. J. Braz. Chem. Soc. 2011, 22, 558–563. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Markowitz, M.A.; Spector, M.S.; Gaber, B.P. Porous Polysilisesquioxanes for the Adsorption of Phenols. Environ. Sci. Technol. 2002, 36, 2515–2518. [Google Scholar] [CrossRef] [PubMed]
- Hatton, B.; Landskron, K.; Whitnall, W.; Perovic, D.; Ozin, G.A. Past, present, and future of periodic mesoporous organosilicas—The PMOs. Acc. Chem. Res. 2005, 38, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Jayasundera, S.; Burleigh, M.C.; Zeinali, M.; Spector, M.S.; Miller, J.B.; Yan, W.F.; Dai, S.; Markowitz, M.A. Organosilica copolymers for the adsorption and separation of multiple pollutants. J. Phys. Chem. B 2005, 109, 9198–9201. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Shi, X.; Liu, J.; Yang, Q. Synthesis of SBA-15 type mesoporous organosilicas with diethylenebenzene in the framework and post-synthetic framework modification. Microporous Mesoporous Mater. 2007, 98, 220–226. [Google Scholar] [CrossRef]
- Li, C.M.; Liu, J.; Shi, X.; Yang, J.; Yang, Q.H. Periodic mesoporous organosilicas with 1,4-diethylenebenzene in the mesoporous wall: Synthesis, characterization, and bioadsorption properties. J. Phys. Chem. C 2007, 111, 10948–10954. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct syntheses of ordered sba-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Melde, B.J.; Holland, B.T.; Blanford, C.F.; Stein, A. Mesoporous sieves with unified hybrid inorganic/organic frameworks. Chem. Mater. 1999, 11, 3302–3308. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q. Modification of MCM-41 by surface silylation with trimethylchlorosilane and adsorption study. J. Phys. Chem. B 1998, 102, 1556–1561. [Google Scholar] [CrossRef]
- Loy, D.A.; Shea, K.J. Bridged Polysilsesquioxanes. Highly porous hybrid organic-inorganic materials. Chem. Rev. 1995, 95, 1431–1442. [Google Scholar] [CrossRef]
- Huo, Q.S.; Margolese, D.I.; Stucky, G.D. Surfactant control of phases in the synthesis of mesoporous silica-based materials. Chem. Mater. 1996, 8, 1147–1160. [Google Scholar] [CrossRef]
- Johnson, B.J.; Melde, B.J.; Charles, P.T.; Dinderman, M.A.; Malanoski, A.P.; Leska, I.A.; Qadri, S.A. Macroporous silica for concentration of nitroenergetic targets. Talanta 2010, 81, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kobayashi, Y.; Amatani, T.; Hirao, K.; Kodaira, T. Spontaneous formation of hierarchical macro-mesoporous ethane-silica monolith. Chem. Mater. 2004, 16, 3652–3658. [Google Scholar] [CrossRef]
- Melde, B.J.; Johnson, B.J.; Dinderman, M.A.; Deschamps, J.R. Macroporous periodic mesoporous organosilicas with diethylbenzene bridging groups. Microporous Mesoporous Mater. 2010, 130, 180–188. [Google Scholar] [CrossRef]
- Chen, B.; Wang, W.S.; Liu, X.; Xue, W.M.; Ma, X.X.; Chen, G.L.; Yu, Q.S.; Li, R. Adsorption study of glycerol in biodiesel on the sulfonated adsorbent. Ind. Eng. Chem. Res. 2012, 51, 12933–12939. [Google Scholar] [CrossRef]
- Nakanishi, K.; Amatani, T.; Yano, S.; Kodaria, T. Multiscale templating of siloxane gels via polymerization-induced phase separation. Chem. Mater. 2008, 20, 1108–1115. [Google Scholar] [CrossRef]
- Johnson, B.J.; Leska, I.A.; Melde, B.J.; Siefert, R.L.; Malanoski, A.P.; Moore, M.H.; Taft, J.R.; Deschamps, J.R. Extraction of perchlorate using porous organosilicate materials. Materials 2013, 6, 1403–1419. [Google Scholar] [CrossRef]
- Johnson, B.J.; Melde, B.J.; Peterson, G.W.; Schindler, B.J.; Jones, P. Functionalized organosilicate materials for irritant gas removal. Chem. Eng. Sci. 2012, 68, 376–382. [Google Scholar] [CrossRef]
- International, A. ASTM D6584-13e1, Standard Test Method for Determination of Total Monoglycerides, Total Diglycerides, Total Triglycerides, and Free and Total Glycerin in B-100 Biodiesel Methyl Esters by Gas Chromatography; ASTM: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Johnson, B.J.; Malanoski, A.P.; Leska, I.A.; Melde, B.J.; Taft, J.R.; Dinderman, M.A.; Deschamps, J.R. Adsorption of organophosphates from solution by porous organosilicates: Capillary phase-separation. Microporous Mesoporous Mater. 2014, 195, 154–160. [Google Scholar] [CrossRef]
- Johnson, B.J.; Melde, B.J.; Charles, P.T.; Cardona, D.C.; Dinderman, M.A.; Malanoski, A.P.; Qadri, S.B. Imprinted nanoporous organosilicas for selective adsorption of nitroenergetic targets. Langmuir 2008, 24, 9024–9029. [Google Scholar] [CrossRef] [PubMed]
- Hankova, L.; Holub, L.; Meng, X.J.; Xiao, F.S.; Jerabek, K. Role of water as a coporogen in the synthesis of mesoporous poly(divinylbenzenes). J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Dou, B.J.; Hu, Q.; Li, J.J.; Qiao, S.Z.; Hao, Z.P. Adsorption performance of VOCs in ordered mesoporous silicas with different pore structures and surface chemistry. J. Hazard. Mater. 2011, 186, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Dube, D.; Rat, M.; Beland, F.; Kaliaguine, S. Sulfonic acid functionalized periodic mesostructured organosilica as heterogeneous catalyst. Microporous Mesoporous Mater. 2008, 111, 596–603. [Google Scholar] [CrossRef]
- Johnson, B.J.; Melde, B.J.; Leska, I.A.; Charles, P.T.; Hewitt, A.D. Solid-phase extraction using hierarchical organosilicates for enhanced detection of nitroenergetic targets. J. Environ. Monit. 2011, 13, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Hochberg, M.E.; Rand, T.A.; Resh, V.H.; Krauss, J. Author sequence and credit for contributions in multiauthored publications. PLoS Biol. 2007, 5, e18. [Google Scholar] [CrossRef] [PubMed]










| Scaffold Description | Material | Type | Grafted Phenyl Groups | Sulfonation |
|---|---|---|---|---|
| Silicate sorbent based on tetramethyl orthosilicate, 0.65 g mesitylene used in synthesis | S65 | -- | No | none |
| S65-Ph(1) | 1 | Yes | standard | |
| Silicate sorbent based on tetramethyl orthosilicate, 0.85 g mesitylene used in synthesis | S85 | -- | No | none |
| S85-Ph(1) | 1 | Yes | standard | |
| Ethane-bridged organosilicate, 0.5 g mesitylene used in synthesis | E25 | -- | No | none |
| E25-Ph(2) | 2 | Yes | standard | |
| Ethane-bridged organosilicate, 1.0 g mesitylene used in synthesis | E50 | -- | No | none |
| E50-Ph(2) | 2 | Yes | standard | |
| Organosilicate with mixed ethane and diethylbenzene bridging groups | ED11 | -- | No | none |
| ED11-Ph | -- | Yes | none | |
| ED11a(4) | 4 | No | standard | |
| ED11-Ph1(3) | 3 | Yes | standard | |
| ED11-Ph2(3) | 3 | Yes | standard | |
| Diethylbenzene-bridged organosilicate | D13 | -- | No | none |
| D13a(6) | 6 | No | standard | |
| D13b(6) | 6 | No | dilute | |
| D13c(6) | 6 | No | standard | |
| D13-Ph1(5) | 5 | Yes | standard | |
| D13-Ph2(5) | 5 | Yes | dilute | |
| Divinylbenzene resin, synthesized with water | PDVB-2 | -- | No | standard |
| Divinylbenzene resin, synthesized with no water | PDVB-3 | -- | No | standard |
| Material | Type | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (Å) | qs | k (1/mg) | n | Chi2 | Std. Error | |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/g | μg/m2 | |||||||||
| S65 † | -- | 591 | 0.675 | 65 | -- | -- | -- | -- | -- | -- |
| S85 † | -- | 552 | 0.716 | 75 | -- | -- | -- | -- | -- | -- |
| S65-Ph(1) | 1 | 525 | 0.542 | 56 | 37.3 | 72.4 | 2.78 | 1 | 0.334 | 0.160 |
| S85-Ph(1) | 1 | 578 | 0.626 | 64 | 24.5 | 42.4 | 1.92 | 1 | 0.008 | 0.024 |
| E25 † | -- | 1088 | 1.32 | 88 | -- | -- | -- | -- | -- | -- |
| E50 † | -- | 1143 | 1.050 | 76 * | -- | -- | -- | -- | -- | -- |
| E25-Ph(2) | 2 | 803 | 0.740 | 64 | 12.0 | 14.9 | 4.78 | 1 | 0.018 | 0.361 |
| E50-Ph(2) | 2 | 883 | 0.664 | -- * | 5.2 | 5.90 | 10.8 | 1 | 0.023 | 0.042 |
| ED11 † | -- | 711 | 0.817 | 55 | 38.8 | 55.4 | 0.461 | 1 | 0.001 | 0.012 |
| ED11-Ph † | -- | 498 | 0.560 | 57 | -- | -- | -- | -- | -- | -- |
| ED11a(4) | 4 | 87 | 0.111 | 55 | 18.0 | 207 | 0.920 | 1 | 0.022 | 0.047 |
| ED11-Ph1(3) | 3 | 147 | 0.151 | 48 | 21.3 | 145 | 13.5 | 1 | 0.057 | 0.069 |
| ED11-Ph2(3) | 3 | 27 | 0.047 | 50 | 12.2 | 452 | 24.0 | 1 | 0.007 | 0.031 |
| D13 † | -- | 427 | 0.642 | 74 | 0.006 | 13.7 | 0.011 | 1 | 0.019 | 0.014 |
| D13a(6) | 6 | 3.1 | 0.005 | -- * | 16.4 | 5290 | 4.56 | 1 | 0.031 | 0.016 |
| D13b(6) | 6 | 337 | 0.536 | 75 | 273 | 810 | 1.96 | 1 | 0.001 | 0.008 |
| D13c(6) | 6 | 3.2 | 0.011 | -- * | 16.6 | 5188 | 4.45 | 1 | 0.016 | 0.037 |
| D13-Ph1(5) | 5 | 4.0 | 0.007 | -- * | 10.8 | 2700 | 3.95 | 1 | 0.081 | 0.082 |
| D13-Ph2(5) | 5 | 310 | 0.497 | 75 | 552 | 1780 | 4.15 | 1 | 0.058 | 0.020 |
| PDVB-2 | -- | 405 | 0.564 | 126 | 4.7 | 11.6 | 26.8 | 1 | 0.022 | 0.028 |
| PDVB-3 | -- | 336 | 0.375 | 65 | 11.2 | 33.3 | 15.8 | 1 | 0.277 | 0.141 |
| BD-ZorbX γ | -- | 0.48 | <0.01 | -- | 4.8 | -- | 6.01 | 1 | 0.021 | 0.046 |
| DW-R10 γ | -- | -- | -- | -- | 2.8 | -- | 7.20 | 1 | 0.007 | 0.031 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, B.J.; Melde, B.J.; Moore, M.H.; Malanoski, A.P.; Taft, J.R. Improving Sorbents for Glycerol Capture in Biodiesel Refinement. Materials 2017, 10, 682. https://doi.org/10.3390/ma10060682
Johnson BJ, Melde BJ, Moore MH, Malanoski AP, Taft JR. Improving Sorbents for Glycerol Capture in Biodiesel Refinement. Materials. 2017; 10(6):682. https://doi.org/10.3390/ma10060682
Chicago/Turabian StyleJohnson, Brandy J., Brian J. Melde, Martin H. Moore, Anthony P. Malanoski, and Jenna R. Taft. 2017. "Improving Sorbents for Glycerol Capture in Biodiesel Refinement" Materials 10, no. 6: 682. https://doi.org/10.3390/ma10060682
APA StyleJohnson, B. J., Melde, B. J., Moore, M. H., Malanoski, A. P., & Taft, J. R. (2017). Improving Sorbents for Glycerol Capture in Biodiesel Refinement. Materials, 10(6), 682. https://doi.org/10.3390/ma10060682