2.1.1. Antibacterial Performance of the Nonwoven Coated Samples Using BS EN ISO 20743:2007
With reference to BS EN ISO 20743:2007 [
34], the results in
Table 2 and
Table 3 report the average reduction of bacteria in colony forming units (CFU), for either
S. aureus or
Klebsiella pneumoniae (
K. pneumoniae), respectively. For the MH coated and synthetic MGO-coated nonwovens, a 100 CFU% reduction in bacteria was achieved for all samples where the calculated concentration of MGO ranged from 0.0054 mg cm
−2 to 0.0170 mg cm
−2 regardless of the strain tested. Interestingly, an average reduction of 97 CFU% in bacteria was still reported for the nonwoven control against
S. aureus (
Table 2), whilst considerably high growth (−252 CFU%) was reported when the same sample was challenged with
K. pneumonia (
Table 3). This latter effect was still observed in the case of the woven polyester control following contact with either
S. aureus or
K. pneumoniae (−22,438 CFU% and −5635 CFU%).
Previously, it has been reported that TENCEL
® or lyocell fibres are able to reduce the growth of
S. aureus considerably when compared with synthetic fibres such as polypropylene, polyester and polyacrylate [
35]. The previous study showed that the synthetic samples exhibited 100 to 1000 times higher bacteria growth when compared with lyocell. It is conceivable that the reduced growth of bacteria observed with lyocell fibres is associated with the behaviour of the fibres in water. In the case of the synthetic fibres, there is limited penetration of water into the fibres and interactions are mainly at the surface which is fully accessible to bacterial organisms. However, because of the nanofibrillar structure of lyocell fibres, water can be absorbed into the micro capillaries inside the fibre, such that there is a reduced life sustaining environment for the bacteria to thrive [
35]. It was reported that approximately 1,333,000 nanofibrils with a diameter of 10 nm are apparent in a single TENCEL fibre, thus contributing to the highly absorbent characteristic nature of the fibre [
36]. This behaviour is therefore a likely explanation as to why a reduced bacterial count (97 CFU%) was observed for the NW control in the case of
S. aureus in the present study. Following these considerations, the thinner peptidoglycan and additional lipopolysaccharide layer present in gram-negative
K. pneumoniae compared to gram-positive
S. aureus [
37] are likely to provide
K. pneumoniae with increased adaptability on hydrated fibres in the experimental conditions investigated, explaining why
K. pneumoniae growth, rather than reduction, was observed in contact with the nonwoven, similarly to the polyester, control (
Table 3).
2.1.2. Antibacterial Performance of the Coated Nonwoven Samples Using BS EN ISO 20645:2004
Table 4 and
Table 5 summarise the results for the NW control and MH- and MGO-coated nonwoven samples against
E. coli and
S. aureus respectively in accordance with BS EN ISO 20645:2004 [
38].
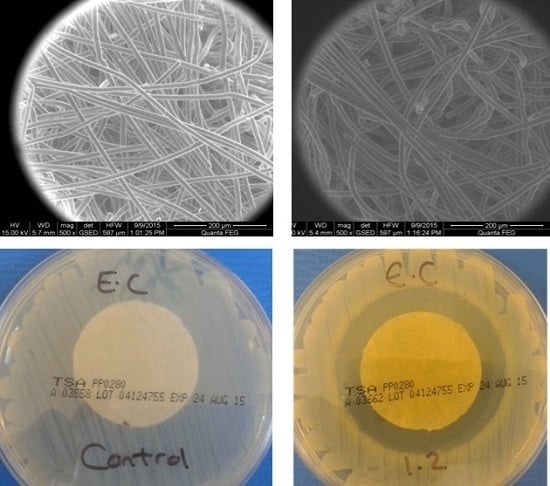
Figure 1 illustrates the influence of the NW control samples on the growth of bacteria.
Figure 2 and
Figure 3 exemplify the effects that the MH- and MGO-coated samples have on the bacterial growth at varying MGO concentrations.
As shown in
Figure 1A,C, no zone of inhibition was apparent with the control samples when tested against both gram-negative
E. coli and gram-positive
S. aureus. Upon the removal of the control samples from the surface of the agar, the contact zone between the sample and the agar presented heavy bacterial growth (
Figure 1B,D). This confirms that the control samples did not exhibit any antibacterial activity. Whilst these observations appear to be in contrast with the results provided in
Table 2, it is important to note that in this case, the samples were directly tested in contact with inoculated agar gels in the absence of simulated wound exudate solution (in contrast to the case of the assay results provided in
Table 2). Here, the bacteria-detrimental fibre-induced water uptake effect was largely marginal, so that high growth of
S. aureus was consequently still observed following application of the nonwoven control sample. The antibacterial effect of the MH and MGO coatings having equivalent MGO concentrations between 0.0054 mg cm
−2 and 0.0170 mg cm
−2 showed no zone of inhibition for
E. coli and
S. aureus. Upon the removal of the MH coated samples from the agar, heavy growth was apparent at an MGO concentration of 0.0054 mg cm
−2 for both
E. coli and
S. aureus (
Figure 2A displays an example of heavy growth). Moderate growth was achieved for both
E. coli and
S. aureus, upon the removal of the MGO-coated nonwovens at equivalent concentrations (an example of moderate growth is shown in
Figure 2B). At an MGO concentration of 0.0170 mg cm
−2, moderate and heavy growth was observed for
E. coli and
S. aureus respectively for the MH-coated samples. However, for the MGO coatings with an equivalent MGO concentration of 0.0170 mg cm
−2, no growth and slight growth was evident against
E. coli and
S. aureus, respectively (examples of slight growth and no growth are shown in
Figure 2C,D). These initial evaluations at a concentration of 0.0054 mg cm
−2 suggest that an insufficient antibacterial effect was achieved for both MH and MGO coatings. At a concentration of 0.0170 mg cm
−2, limited efficacy was observed for the MH coatings. However, for the MGO coatings with an MGO concentration of 0.0170 mg cm
−2, the antibacterial effect was shown to improve slightly and a good antibacterial effect and a limit of efficiency was achieved for both
E. coli and
S. aureus respectively.
It is important to note that where no growth or inhibition zone was apparent, a good antibacterial effect may still be observed. This may be linked to the diffusion rate of the active compound from the fabric [
38] to the agar plate and the affinity of the fibres for moisture. Thus, it is likely that, within the time frames investigated in this study, the hygroscopic, crystalline nanofibrils of the TENCEL
® fibres [
35] retain the MGO and honey coating, thereby limiting the diffusion of MGO into the agar at these MGO concentrations. This situation may well be expected in this case, given that no additional simulated wound exudate solution was applied. The minimal swelling of the fibres expected following contact with the agar plate may well be directly related to a decreased MGO diffusion. This hypothesis is supported when comparing data obtained in exudate-free conditions with the ones presented in
Table 2 and
Table 3, where complete bacteria killing was observed with the same MGO concentrations following the addition of simulated wound exudate solution. As the MGO concentration increases to between 0.1 mg cm
−2 and 1.2 mg cm
−2, a good antibacterial effect is observed with both MH and MGO in all cases (
Table 4 and
Table 5). For the MH coated samples, mean zones of inhibition of 0–1 mm were apparent against both
E. coli and
S. aureus at concentrations between 0.1 mg cm
−2 and 0.2 mg cm
−2.
Figure 3B displays an example of an inhibition zone from 0–1 mm. As the concentration of MGO doubled to 0.2 mg cm
−2, the mean zone of inhibition for
E. coli and
S. aureus increased to achieve a mean zone of >1 mm. An example of a mean zone of >1 mm can be seen in
Figure 3C.
Conversely, the MGO coatings did not show a clear zone of inhibition until a concentration of 0.4 mg cm
−2 was reached for
E. coli and 0.8 mg cm
−2 for
S. aureus. Below these concentrations, no evidence of bacterial growth was observed upon the removal of the samples, resulting in a good antibacterial effect. However, a partial zone of inhibition was formed around the samples, as presented in
Figure 3D, suggesting that the TENCEL
® fibres still retained a proportion of the MGO. As the addition of MGO solution increased, the TENCEL
® fibres uptake of, and ability to retain, the MGO was reduced. This is expected to encourage greater diffusion of MGO into the bacteria-seeded agar, resulting in a clear zone of inhibition.
Figure 4 shows FEGSEM images of the dry (
Figure 4A), MGO-coated (
Figure 4B) and the MH-coated (
Figure 4C,D) TENCEL
® fibres. It is apparent that the MGO-coated TENCEL
® fibres (
Figure 4B) have a similar appearance to the dry TENCEL
® fibres as seen in
Figure 4A, confirming that the liquid phase coating has been absorbed and retained by the fibres. The MH-coated TENCEL
® fibres appear mainly occluded by the honey coating (
Figure 4C,D), and some protruding fibres exhibit a globular surface coating due to the MH. These images provided further evidence that the MH is freely available on the surface of the TENCEL
® fibres, such that direct contact with the bacteria agar can be anticipated. It is also likely that during incubation at 37 °C, the MH coating will soften and allow greater diffusion into the bacteria-seeded agar from the fibres. Previous studies have reported that temperature has a direct influence on the viscosity of honey [
39,
40,
41] such that as the temperature increases the viscosity falls due to reduced hydrodynamic forces and reduced molecular interaction [
41]. The viscosity measurements of the MH obtained during this study confirmed this temperature dependency, with a decreased viscosity of 17,800 cP being obtained at 37 °C ± 2 °C rising to 21,800 cP at 25 °C ± 2 °C. This data therefore suggests that the MH coating is more likely to migrate freely into the bacteria-seeded agar during testing, as a result of low viscosity at 37 °C.
A previous investigation of the antibacterial activity of MH and MGO in a liquid form reported that higher levels of MGO alone were required to inhibit the growth of
P. aeroginosa when compared with MH where equivalent MGO concentrations were apparent [
42]. Secondly, the presence of hydrogen peroxide in the MH may contribute to the heightened antibacterial effect [
42,
43,
44,
45].
Comparing the results obtained using both antibacterial methods, the concentration of MGO required to produce an antibacterial effect was found to be slightly lower (0.0054 mg cm
2) when assessed according to BS EN ISO 20743:2007, compared to concentrations between 0.0170 mg cm
2 and 0.1 mg cm
2 for
E. coli and
S. aureus respectively when using BS EN ISO 20645:2004. The lower MGO concentration achieved using BS EN ISO 20743:2007 may be attributed to the addition of the liquid used to simulate wound exudate (SCDLP) solution during testing [
34]. This would result in the TENCEL
® fibres being exposed to higher moisture content, which could encourage the hydration of the fibres and facilitate extraction of the MGO. In the case of BS EN ISO 20645:2004 an insufficient moisture content is available to initiate the diffusion of the MGO from the fibres [
38]. It is only when the nonwoven samples become increasingly hydrated that the diffusion of MGO into the agar is promoted.