Synthesis and Characterization of a Heterometallic Extended Architecture Based on a Manganese(II)-Substituted Sandwich-Type Polyoxotungstate
Abstract
:1. Introduction
- (a)
- (b)
- (c)
- (d)
- (e)
2. Experimental
2.1. Synthesis
2.2. Methods
2.2.1. Crystallography
2.2.2. Magnetic Measurements
3. Results and Discussion
3.1. Synthesis
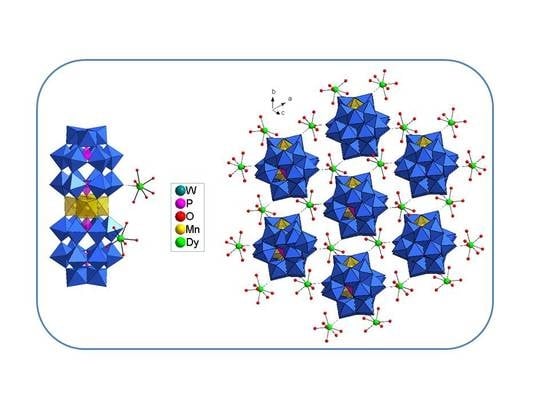
3.2. Structure
3.3. Characterizations
4. Magnetic Properties
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Long, D.L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef] [PubMed]
- Bassil, B.S.; Kortz, U. Recent advances in lanthanide-containing polyoxotungstates. Z. Anorg. Allg. Chem. 2010, 636, 2222–2231. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F. Transition metal complexes that catalyze oxygen formation from water: 1979–2010. Coord. Chem. Rev. 2012, 256, 1115–1136. [Google Scholar] [CrossRef]
- Ruttinger, W.; Dismukes, G.C. Synthetic water-oxidation catalysts for artificial photosynthetic water oxidation. Chem. Rev. 1997, 97, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Chen, L.; Yang, G. Research progress on polyoxometalate-based transition-metal–rare-earth heterometallic derived materials: Synthetic strategies, structural overview and functional applications. Chem. Commun. 2016, 52, 4418–4445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, Q.; Chen, L.; Zhao, J. A brief review of the crucial progress on heterometallic polyoxotungstates in the past decade. CrystEngComm 2016, 18, 842–862. [Google Scholar] [CrossRef]
- Ibrahim, M.; Dickman, M.H.; Suchopar, A.; Kortz, U. Large cation- Anion materials based on trinuclear ruthenium(III) salts of Keggin and Wells-Dawson anions having water-filled channels. Inorg. Chem. 2009, 48, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Oms, O.; Dolbecq, A.; Mialane, P. Diversity in structures and properties of 3d-incorporating polyoxotungstates. Chem. Soc. Rev. 2012, 41, 7497–7536. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Lan, Y.; Bassil, B.S.; Xiang, Y.; Suchopar, A.; Powell, A.K.; Kortz, U. Hexadecacobalt(II)-containing polyoxometalate-based single-molecule magnet. Angew. Chem. Int. Ed. 2011, 50, 4708–4711. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Haider, A.; Xiang, Y.; Bassil, B.S.; Carey, A.M.; Rullik, L.; Jameson, G.B.; Doungmene, F.; Mbomekallé, I.M.; De Oliveira, P.; et al. Tetradecanuclear iron(III)-oxo nanoclusters stabilized by trilacunary heteropolyanions. Inorg. Chem. 2015, 54, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Ibrahim, M.; Bassil, B.S.; Carey, A.M.; Viet, A.N.; Xing, X.; Ayass, W.W.; Miñambres, J.F.; Liu, R.; Zhang, G.; et al. Mixed-valent Mn16-containing heteropolyanions: Tuning of oxidation state and associated physicochemical properties. Inorg. Chem. 2016, 55, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Bassil, B.S.; Ibrahim, M.; Al-Oweini, R.; Asano, M.; Wang, Z.; Van Tol, J.; Dalal, N.S.; Choi, K.Y.; Ngo Biboum, R.; Keita, B.; et al. A planar {Mn19(OH)12}26+ unit incorporated in a 60-tungsto-6-silicate polyanion. Angew. Chem. Int. Ed. 2011, 50, 5961–5964. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Conrad, F.; Patzke, G.R. A gadolinium-bridged polytungstoarsenate(III) nanocluster: [Gd8As12W124O432(H2O)22]60−. Angew. Chem. Int. Ed. 2009, 48, 9088–9091. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, S. Heterometallic 3d-4f polyoxometalates: Still an incipient field. Dalton Trans. 2011, 40, 6610–6615. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Mereacre, V.; Leblanc, N.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. Self-assembly of a giant tetrahedral 3 d-4 f single-molecule magnet within a polyoxometalate system. Angew. Chem. Int. Ed. 2015, 54, 15574–15578. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Kögerler, P.; Furukawa, Y.; Speldrich, M.; Luban, M. Molecular growth of a core-shell polyoxometalate. Angew. Chem. Int. Ed. 2011, 50, 5212–5216. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, K.; Dickman, M.H.; Pope, M.T. Self-assembly of supramolecular polyoxometalates: The compact, water-soluble heteropolytungstate anion[As12IIICe16III(H2O)36W148O524]76−. Angew. Chem. Int. Ed. Engl. 1997, 36, 1445–1448. [Google Scholar] [CrossRef]
- Bassil, B.S.; Dickman, M.H.; Römer, I.; Von Der Kammer, B.; Kortz, U. The tungstogermanate [Ce20Ge10W100O376(OH)4(H2O)30]56−: A polyoxometalate containing 20 cerium(III) atoms. Angew. Chem. Int. Ed. 2007, 46, 6192–6195. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Gable, R.W.; Speldrich, M.; Kögerler, P.; Boskovic, C. Polyoxotungstate-encapsulated Gd6 and Yb10 complexes. Chem. Commun. 2009, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Artetxe, B.; Reinoso, S.; San Felices, L.; Gutiérrez-Zorrilla, J.M.; García, J.A.; Haso, F.; Liu, T.; Vicent, C. Crown-shaped tungstogermanates as solvent-controlled dual systems in the formation of vesicle-like assemblies. Chem. A Eur. J. 2015, 21, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Chen, H.; Zhao, J.; Ma, P.; Wang, J. 1-D, 2-D, and 3-D organic-inorganic hybrids assembled from keggin-type polyoxometalates and 3d-4f heterometals. Cryst. Growth Des. 2011, 11, 3769–3777. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, D.; Chen, L.; Ma, P.; Wang, J.; Zhang, J.; Niu, J. Tetrahedral polyoxometalate nanoclusters with tetrameric rare-earth cores and germanotungstate vertexes. Cryst. Growth Des. 2013, 13, 4368–4377. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Li, Y.-Z.; Ji, F.; Yuan, J.; Chen, L.-J.; Yang, G.-Y. Syntheses, structures and electrochemical properties of a class of 1-D double chain polyoxotungstate hybrids [H2dap][Cu(dap)2]0.5[Cu(dap)2(H2O)][Ln(H2O)3(α-GeW11O39)]·3H2O. Dalton Trans. 2014, 43, 5694–5706. [Google Scholar] [CrossRef] [PubMed]
- Compain, J.D.; Mialane, P.; Dolbecq, A.; Mbomekallé, I.M.; Marrot, J.; Sécheresse, F.; Duboc, C.; Rivière, E. Structural, magnetic, EPR, and electrochemical characterizations of a spin-frustrated trinuclear CrIII polyoxometalate and study of its reactivity with lanthanum cations. Inorg. Chem. 2010, 49, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Wang, Y.; Wang, E. An inorganic aggregate based on a sandwich-type polyoxometalate with lanthanide and potassium cations: From 1D chiral ladder-like chains to a 3D open framework. Eur. J. Inorg. Chem. 2007, 2216–2220. [Google Scholar] [CrossRef]
- Fan, L.Y.; Lin, Z.G.; Cao, J.; Hu, C.W. Probing the Self-Assembly Mechanism of Lanthanide-Containing Sandwich-Type Silicotungstates [{Ln(H2O)n}2{Mn4(B-α-SiW9O34)2(H2O)2}]6− Using Time-Resolved Mass Spectrometry and X-ray Crystallography. Inorg. Chem. 2016, 55, 2900–2908. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ma, H.; Zhang, C.; Pang, H.; Li, S.; Liu, H. A 3d–4f heterometallic 3D POMOF based on lacunary Dawson polyoxometalates. Dalton Trans. 2013, 42, 16328–16333. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Moore, E.G.; Speldrich, M.; Kögerler, P.; Boskovic, C. Terbium polyoxometalate organic complexes: Correlation of structure with luminescence properties. Angew. Chem. Int. Ed. 2010, 49, 7702–7705. [Google Scholar] [CrossRef] [PubMed]
- Vonci, M.; Boskovic, C. Polyoxometalate-supported lanthanoid single-molecule magnets. Aust. J. Chem. 2014, 67, 1542–1552. [Google Scholar] [CrossRef]
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the stability of metal-organic frameworks. Adv. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Finke, R.G.; Droege, M.W.; Domaille, P.J. Rational syntheses, characterization, two-dimensional tungsten-183 NMR, and properties of tungstometallophosphates P2W18M4(H2O)2O6810− and P4W30M4(H2O)2O11216− (M = cobalt, copper, zinc). Inorg. Chem. 1987, 26, 3886–3896. [Google Scholar] [CrossRef]
- Gómez-García, C.J.; Borrás-Almenar, J.J.; Coronado, E.; Ouahab, L. Single-crystal X-Ray structure and magnetic properties of the polyoxotungstate complexes Na16[M4(H2O)2(P2W15O56)2]·nH2O (M = MnII, n = 53; M = NiII, n = 52): An antiferromagnetic MnII tetramer and a ferromagnetic NiII tetramer. Inorg. Chem. 1994, 33, 4016–4022. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Yamase, T.; Kobayashi, T.; Sugeta, M.; Naruke, H. Europium(III) luminescence and intramolecular energy transfer studies of polyoxometalloeuropates. J. Phys. Chem. A 1997, 101, 5046–5053. [Google Scholar] [CrossRef]
- Sun, H.; Liu, M.Q.; Zhang, B.J. Two dysprosium complexes based on 8-hydroxyquinoline Schiff base: Structures, luminescence properties and single-molecule magnets behaviors. Inorg. Chim. Acta 2016, 453, 681–686. [Google Scholar] [CrossRef]
- Xu, L.J.; Sun, C.Z.; Xiao, H.; Wu, Y.; Chen, Z.N. Green-light-emitting diodes based on tetrabromide manganese(II) complex through solution process. Adv. Mater. 2017, 29, 2–6. [Google Scholar] [CrossRef] [PubMed]











| Compound | Dy2Mn4-P2W15 |
|---|---|
| Formula | Dy2H146Mn4Na10O185P4W30 |
| Formula weight | 9521.20 |
| Crystal System | Triclinic |
| Space Group | P |
| a/Å | 13.3544(4) |
| b/Å | 14.7907(4) |
| c/Å | 22.5101(6) |
| α/° | 79.938(2) |
| β/° | 76.843(2) |
| γ/° | 65.236(3) |
| V/Å3 | 3915.5(2) |
| Z | 2 |
| T/K | 180(2) |
| Crystal dimensions/mm | 0.11 × 0.06 × 0.02 |
| F(000) | 4248 |
| Dc/Mg m−3 | 4.038 |
| μ(Mo-Kα)/mm−1 | 23.389 |
| Data Measured | 49,270 |
| Unique Data | 17,079 |
| Rint | 0.0427 |
| Data with I ≥ 2σ(I) | 14,193 |
| wR2 (all data) | 0.2055 |
| S (all data) | 1.083 |
| R1 [I ≥ 2σ(I)] | 0.0732 |
| Parameters/Restraints | 1045/29 |
| Biggest diff. peak/hole/eÅ−3 | +4.53/−1.87 |
| FIZ-CSD number | 433863 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.; Moreno-Pineda, E.; Bergfeldt, T.; Anson, C.E.; Powell, A.K. Synthesis and Characterization of a Heterometallic Extended Architecture Based on a Manganese(II)-Substituted Sandwich-Type Polyoxotungstate. Materials 2018, 11, 155. https://doi.org/10.3390/ma11010155
Ibrahim M, Moreno-Pineda E, Bergfeldt T, Anson CE, Powell AK. Synthesis and Characterization of a Heterometallic Extended Architecture Based on a Manganese(II)-Substituted Sandwich-Type Polyoxotungstate. Materials. 2018; 11(1):155. https://doi.org/10.3390/ma11010155
Chicago/Turabian StyleIbrahim, Masooma, Eufemio Moreno-Pineda, Thomas Bergfeldt, Christopher E. Anson, and Annie K. Powell. 2018. "Synthesis and Characterization of a Heterometallic Extended Architecture Based on a Manganese(II)-Substituted Sandwich-Type Polyoxotungstate" Materials 11, no. 1: 155. https://doi.org/10.3390/ma11010155
APA StyleIbrahim, M., Moreno-Pineda, E., Bergfeldt, T., Anson, C. E., & Powell, A. K. (2018). Synthesis and Characterization of a Heterometallic Extended Architecture Based on a Manganese(II)-Substituted Sandwich-Type Polyoxotungstate. Materials, 11(1), 155. https://doi.org/10.3390/ma11010155