Determination of Activation Overpotential during the Nucleation of Hcp-Cobalt Nanowires Synthesized by Potentio-Static Electrochemical Reduction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
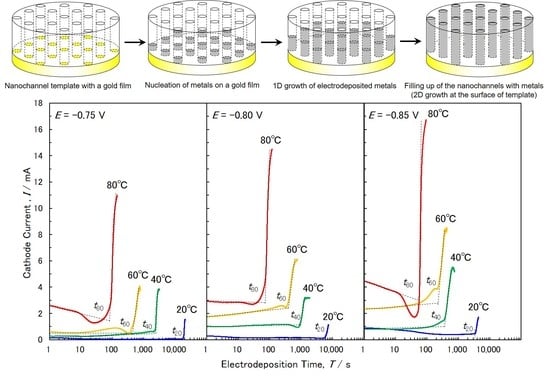
3.1. Electrodeposition of Cobalt Nanowire Arrays
3.2. Texture and Crystallinity of Co Nanowire Arrays
3.3. Magnetization Performance of AAO Nanochannel Films with Co Nanowire Arrays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAO | anodized aluminum oxide |
| FE-SEM | field emission scanning electron microscopy |
| hcp | hexagonal close packed structure |
| VSM | vibrating sample magnetometer |
| SAED | selected area electron diffraction |
| HRTEM | high-resolution transmission electron microscopy |
References
- Li, J.; Pan, M.; Yu, Y.; Ge, H.; Wu, Q. High-performance Sm2Co17 based alloy with enhanced magnetic properties and improved corrosion resistance. Int. J. Electrochem. Sci. 2018, 13, 8897–8904. [Google Scholar] [CrossRef]
- Feng, D.Y.; Liu, Z.W.; Wang, G.; Zheng, Z.G.; Zeng, D.C.; Li, Z.; Zhang, G.Q. Zr and Si co-substitution for SmCo7 alloy with enhanced magnetic properties and improved oxidation and corrosion resistances. J. Alloy Comp. 2014, 610, 341–346. [Google Scholar] [CrossRef]
- García Fernández, J.; Vega Martínez, V.; Thomas, A.; de la Prida Pidal, V.M.; Nielsch, K. Two-Step Magnetization Reversal FORC Fingerprint of Coupled Bi-Segmented Ni/Co Magnetic Nanowire Arrays. Nanomaterials 2018, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Kac, M.; Zarzycki, A.; Kac, S.; Kopec, M.; Perzanowski, M.; Dutkiewicz, E.M.; Suchanek, K.; Maximenko, A.; Marszalek, M. Effect of the template-assisted electrodeposition parameters on the structure and magnetic properties of Co nanowire arrays. Mater. Sci. Eng. B 2016, 211, 75–84. [Google Scholar] [CrossRef]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, X.; Liu, J.P. High energy product developed from cobalt nanowires. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Whitney, T.M.; Searson, P.C.; Jiang, J.S.; Chien, C.L. Fabrication and magnetic properties of arrays of metallic nanowires. Science 1993, 261, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Darques, M.; Piraux, L.; Encinas, A. Influence of the diameter and growth conditions on the magnetic properties of cobalt nanowires. IEEE Trans. Magn. 2005, 41, 3415–3417. [Google Scholar] [CrossRef]
- Yang, P.; An, M.; Su, C.; Wang, F. Fabrication of cobalt nanowires from mixture of 1-ethyl-3-methylimidazolium chloride ionic liquid and ethylene glycol using porous anodic alumina template. Electrochim. Acta 2008, 54, 763–767. [Google Scholar] [CrossRef]
- Han, X.; Liu, Q.; Wang, J.; Li, S.; Ren, Y.; Liu, R.; Li, F. Influence of crystal orientation on magnetic properties of hcp Co nanowire arrays. J. Phys. D Appl. Phys. 2009, 42, 095005. [Google Scholar] [CrossRef]
- Maaz, K.; Karim, S.; Usman, M.; Mumtaz, A.; Liu, J.; Duan, J.L.; Maqbool, M. Effect of crystallographic texture on magnetic characteristics of cobalt nanowires. Nanoscale Res. Lett. 2010, 5, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Prida, V.M.; Vega, V.; Rosa, W.O.; Flores, R.C.; Iglesias, L.; Hernando, B. 2D and 3D ordered arrays of Co magnetic nanowires. J. Magn. Magn. Mater. 2015, 383, 88–93. [Google Scholar] [CrossRef]
- Ghemes, A.; Dragos-Pinzaru, O.; Chiriac, H.; Lupu, N.; Grigoras, M.; Shore, D.; Stadler, B.; Tabakovic, I. Controlled electrodeposition and magnetic properties of Co35Fe65 nanowires with high saturation magnetization. J. Electrochem. Soc. 2017, 164, D13–D22. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Rubino, A.; Pagnanelli, F. Electrodeposition of cobalt nanowires into alumina templates generated by one-step anodization. Electrochim. Acta 2018, 259, 711–722. [Google Scholar] [CrossRef]
- Cattaneo, L.; Franz, S.; Albertini, F.; Ranzieri, P.; Vincenzo, A.; Bestetti, M.; Cavallotti, P.L. Electrodeposition of hexagonal Co nanowires with large magnetocrystalline anisotropy. Electrochim. Acta 2012, 85, 57–65. [Google Scholar] [CrossRef]
- Neetzel, C.; Ohgai, T.; Yanai, T.; Nakano, M.; Fukunaga, H. Uniaxial magnetization performance of Co-Al2O3 nano-composite films electrochemically synthesized from acidic aqueous solution. J. Solid State Electrochem. 2016, 20, 1665–1672. [Google Scholar] [CrossRef]
- Pirota, K.R.; Beron, F.; Zanchet, D.; Rocha, T.C.R.; Navas, D.; Torrejon, J.; Vazquez, M.; Knobel, M. Magnetic and structural properties of fcc/hcp bi-crystalline multilayer Co nanowire arrays prepared by controlled electroplating. J. Appl. Phys. 2011, 109, 083919. [Google Scholar] [CrossRef]
- Vivas, L.G.; Vazquez, M.; Vega, V.; Garcia, J.; Rosa, W.O.; Real, R.P.; Prida, V.M. Temperature dependent magnetization in Co-base nanowire arrays: Role of crystalline anisotropy. J. Appl. Phys. 2012, 111, 07A325. [Google Scholar] [CrossRef]
- Kaur, D.; Chaudhary, S.; Pandya, D.K.; Gupta, R.; Kotnala, R.K. Magnetization reversal studies in structurally tailored cobalt nanowires. J. Magn. Magn. Mater. 2013, 344, 72–78. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Soltanian, S.; Koohbor, M.; Salimi, A.; Servati, P. Modification of microstructure and magnetic properties of electrodeposited Co nanowire arrays: A study of the effect of external magnetic field, electrolyte acidity and annealing process. Mater. Chem. Phys. 2015, 160, 389–397. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Shekhar, R. Crystal anisotropy induced temperature dependent magnetization in cobalt nanowires electrodeposited within alumina template. J. Magn. Magn. Mater. 2014, 349, 21–26. [Google Scholar] [CrossRef]
- Saeki, R.; Ohgai, T. Effect of growth rate on the crystal orientation and magnetization performance of cobalt nanocrystal arrays electrodeposited from aqueous solution. Nanomaterials 2018, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Ohgai, T.; Tanaka, Y.; Fujimaru, T. Soft magnetic properties of Ni–Cr and Co–Cr alloy thin films electrodeposited from aqueous solutions containing trivalent chromium ions and glycine. J. Appl. Electrochem. 2012, 42, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Blanco, S.; Vargas, R.; Mostany, J.; Borrás, C.; Scharifker, B.R. Modeling the Growth of Nanowire Arrays in Porous Membrane Templates. J. Electrochem. Soc. 2014, 161, E3341–E3347. [Google Scholar] [CrossRef]
- Ohgai, T.; Enculescu, I.; Zet, C.; Westerberg, L.; Hjort, K.; Spohr, R.; Neumann, R. Magneto-sensitive nickel nanowires fabricated by electrodeposition into multi- and single-ion track templates. J. Appl. Electrochem. 2006, 36, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Barriga-Castro, E.D.; Garcıa, J.; Mendoza-Resendez, R.; Prida, V.M.; Luna, C. Pseudo-monocrystalline properties of cylindrical nanowires confinedly grown by electrodeposition in nanoporous alumina templates. RSC Adv. 2017, 7, 13817–13826. [Google Scholar] [CrossRef] [Green Version]
- Ohgai, T.; Shimono, R.; Saitoh, H.; Hayashi, Y. Structure and soft magnetic properties of Fe-N thin films RF-sputtered on heated substrate. Mater. Trans. JIM 1997, 38, 503–507. [Google Scholar] [CrossRef]
- Fernandez-Roldan, J.A.; Chrischon, D.; Dorneles, L.S.; Chubykalo-Fesenko, O.; Vazquez, M.; Bran, C. A comparative study of magnetic properties of large diameter Co nanowires and nanotubes. Nanomaterials 2018, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, W.; Sun, H.; Guo, L.; Sun, J. Growth mechanism and magnetic properties of Co nanowire arrays by AC electrodeposition. J. Magn. Magn. Mater. 2018, 468, 188–192. [Google Scholar] [CrossRef]
- Khan, H.R.; Petrikowski, K. Magnetic and structural properties of the electrochemically deposited arrays of Co and CoFe nanowires. J. Magn. Magn. Mater. 2002, 249, 458–461. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, R.; Ohgai, T. Determination of Activation Overpotential during the Nucleation of Hcp-Cobalt Nanowires Synthesized by Potentio-Static Electrochemical Reduction. Materials 2018, 11, 2355. https://doi.org/10.3390/ma11122355
Saeki R, Ohgai T. Determination of Activation Overpotential during the Nucleation of Hcp-Cobalt Nanowires Synthesized by Potentio-Static Electrochemical Reduction. Materials. 2018; 11(12):2355. https://doi.org/10.3390/ma11122355
Chicago/Turabian StyleSaeki, Ryusei, and Takeshi Ohgai. 2018. "Determination of Activation Overpotential during the Nucleation of Hcp-Cobalt Nanowires Synthesized by Potentio-Static Electrochemical Reduction" Materials 11, no. 12: 2355. https://doi.org/10.3390/ma11122355
APA StyleSaeki, R., & Ohgai, T. (2018). Determination of Activation Overpotential during the Nucleation of Hcp-Cobalt Nanowires Synthesized by Potentio-Static Electrochemical Reduction. Materials, 11(12), 2355. https://doi.org/10.3390/ma11122355