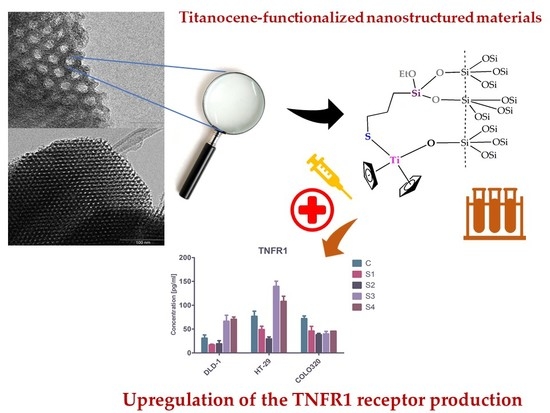
Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Metallodrug-Functionalized Nanostructured Materials
2.1.1. X-ray Fluorescence
2.1.2. Powder X-ray Diffraction
2.1.3. N2 Adsorption-Desorption Isotherms
2.1.4. Solid-State NMR Spectroscopy
2.1.5. Thermogravimetry
2.1.6. IR and UV Spectroscopy
2.1.7. SEM and TEM
2.2. Qualitative Study of the Interactions with DNA
2.3. Cell Growth Inhibition
2.4. Effect on Intracellular Metabolic Activity
2.5. Effect on Inflammatory Processes
2.6. TNFR1
2.7. Study of the Titanium Release
3. Materials and Methods
3.1. General Conditions
3.2. General Conditions on the Synthesis and Characterization of the Titanocene Complexes
3.3. General Conditions for the Characterization of the Materials
3.4. Synthesis of SBA-15
3.5. Synthesis of SBA-15/[Ti(η5-C5H5)2Cl2] (S1)
3.6. Synthesis of SBA-15/[Ti(η5-C5H5)(η5-C5H4Pri)Cl2] (S2)
3.7. Synthesis of SBA-15/[Ti(η5-C5H5)(η5-C5H4SiMe3)Cl2] (S3)
3.8. Synthesis of SBA-15/[Ti(η5-C5H5)2{SCH2CH2CH2Si(OEt)3}2] (S4)
3.9. Ti-Release Studies
3.10. DNA-Binding Studies
3.11. Biological Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Statement
References
- Rosenberg, B.; Camp, L.V. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Spreckelmeyer, S.; Orvig, C.; Casini, A. Cellular Transport Mechanisms of Cytotoxic Metallodrugs: An Overview beyond Cisplatin. Molecules 2014, 19, 15584–15610. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Casini, A. Mass spectrometry as a powerful tool to study therapeutic metallodrugs speciation mechanisms: Current frontiers and perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar] [CrossRef]
- Kiss, T.; Enyedy, E.A.; Jakusch, T. Development of the application of speciation in chemistry. Coord. Chem. Rev. 2017, 352, 401–423. [Google Scholar] [CrossRef]
- Wani, W.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured Materials Functionalized with Metal Complexes: In Search of Alternatives for Administering Anticancer Metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. A short overview on the biomedical applications of silica, alumina and calcium phosphate-based nanostructured materials. Curr. Med. Chem. 2016, 23, 4450–4467. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Z.; Sierra, I.; Prashar, S.; Del Hierro, I.; Fajardo, M.; Juranić, D.Z.; Kaluđerović, G.N. A New Generation of Anticancer Drugs: Mesoporous Materials Modified with Titanocene Complexes. Chem. Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; Del Hierro, I.; Žižak, Z.; Juranić, D.Z.; Fajardo, M.; Gómez-Ruiz, S. Study of the influence of the metal complex on the cytotoxic activity of titanocene-functionalized mesoporous materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Žižak, Z.; Juranić, D.Z.; Gómez-Ruiz, S. Improvement of cytotoxicity of titanocene-functionalized mesoporous materials by the increase of the titanium content. Dalton Trans. 2010, 39, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; Del Hierro, I.; Kaluđerović, G.N. Study of the cytotoxicity and particle action in human cancer cells of titanocene-functionalized materials with potential application against tumors. J. Inorg. Biochem. 2012, 116, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anticancer Applications of Titanocene-Functionalized Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem. Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Chicca, A.; Gertsch, J.; Pinar, A.B.; Gómez-Ruiz, S. Ether-substituted group 4 metallocene complexes: Cytostatic effects and applications in ethylene polymerization. Organometallics 2015, 34, 2522–2532. [Google Scholar] [CrossRef]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-Loaded Mesoporous Silica as a Biocompatible Strategy in Cancer Treatment. Angew. Chem. Int. Ed. 2014, 53, 5982–5987. [Google Scholar] [CrossRef] [PubMed]
- Bensing, C.; Mojić, M.; Gómez-Ruiz, S.; Carralero, S.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Evaluation of functionalized mesoporous silica SBA-15 as a carrier system for Ph3Sn(CH2)3OH against the A2780 ovarian carcinoma cell line. Dalton Trans. 2016, 45, 18984–18993. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. Anticancer Applications and Recent Investigations of Metallodrugs Based on Gallium, Tin and Titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef]
- Cini, M.; Bradshaw, T.N.; Woodward, S. Using titanium complexes to defeat cancer: The view from the shoulders of titans. Chem. Soc. Rev. 2017, 46, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Elegbede, J.A.; Carpera, S.W. Methyl jasmonate decreases membrane fluidity and induces apoptosis via tumor necrosis factor receptor 1 in breast cancer cells. Anticancer Drugs 2008, 19, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, L.C.; Stoop, A.A.; Negm, O.H.; Radford, P.M.; Tighe, P.J.; Todd, I. Tumour necrosis factor receptor I blockade shows that TNF-dependent and TNF-independent mechanisms synergise in TNF receptor associated periodic syndrome. Eur. J. Immunol. 2015, 45, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Kim, E.C.; Han, S.B.; Yoon, D.Y.; Hong, J.T. IL-32α suppresses colorectal cancer development via TNFR1-mediated death signaling. Oncotarget 2015, 6, 9061–9072. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Hu, X.; Liu, S.; He, B. Prognostic and Therapeutic Values of Tumor Necrosis Factor-Alpha in Hepatocellular Carcinoma. Med. Sci. Monit. 2016, 22, 3694–3704. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–620. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kathiravan, A.; Renganathan, R. Photoinduced interactions between colloidal TiO2 nanoparticles and calf thymus-DNA. Polyhedron 2009, 28, 1374–1378. [Google Scholar] [CrossRef]
- Sablina, A.A.; Chumakov, P.M.; Kopnin, B.P. Tumor Suppressor p53 and Its Homologue p73α Affect Cell Migration. J. Biol. Chem. 2003, 278, 27362–27371. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Dolznig, H.; Rupp, C.; Puri, C.; Haslinger, C.; Schweifer, N.; Wieser, E.; Kerjaschki, D.; Garin-Chesa, P. Modeling colon adenocarcinomas in vitro a 3D co-culture system induces cancer-relevant pathways upon tumor cell and stromal fibroblast interaction. Am. J. Pathol. 2011, 179, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe-Cetean, S.; Cainap, C.; Oprean, L.; Hangan, A.; Virag, P.; Fischer-Fodor, E.; Gherman, A.; Cainap, S.; Constantin, A.M.; Laszlo, I.; et al. Platinum derivatives: A multidisciplinary approach. J. BUON 2017, 22, 568–577. [Google Scholar] [PubMed]
- Miklášová, N.; Fischer-Fodor, E.; Mikláš, R.; Kucková, L.; Kožíšek, J.; Liptaj, T.; Soritau, O.; Valentová, J.; Devínsky, F. Synthesis and characterization of new biologically active palladium(II) complexes with (1E,6E)-1,7-bis(3,4-diethoxyphenyl)-1,6-heptadiene-3,5-dione. Inorg. Chem. Commun. 2014, 46, 229–233. [Google Scholar] [CrossRef]
- Doyle, M.V.; Brindley, L.; Kawasaki, E.; Larrick, J. High level human interleukin 1 production by a hepatoma cell line. Biochem. Biophys. Res. Commun. 1985, 130, 768–773. [Google Scholar] [CrossRef]
- Voronov, E.; Apte, R.N. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015, 8, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.S.; Kuratnik, A.; Scocchera, E.W.; Wright, D.L.; Giardina, C. Identification of novel compounds that enhance colon cancer cell sensitivity to inflammatory apoptotic ligands. Cancer Biol. Ther. 2013, 14, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Köpf, H. Antitumor metallocenes: New developments and toxicologic features. Anticancer Res. 1986, 6, 227–233. [Google Scholar] [PubMed]
- Fischer, R.; Kontermann, R.E.; Maier, O. Targeting sTNF/TNFR1 Signaling as a New Therapeutic Strategy. Antibodies 2015, 4, 48–70. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Clark, R.J.H.; Moorhouse, S. Reactions of trimethylsilylcyclopentadiene derivatives with titanium, niobium, and tantalum halides. J. Chem. Soc. Dalton Trans. 1980, 7, 1156–1160. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.; Stucky, G. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Pérez-Pariente, J.; Vallet-Regí, M. Influence of pore size of MCM-41 matrices on drug delivery rate. Microporous Mesoporous Mater. 2004, 68, 105–109. [Google Scholar] [CrossRef]
- Marmur, J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 1961, 3, 208–218. [Google Scholar] [CrossRef]
- Reichmann, M.E.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Tomuleasa, C.; Soritau, O.; Orza, A.; Dudea, M.; Petrushev, B.; Mosteanu, O.; Susman, S.; Florea, A.; Pall, E.; Aldea, M.; et al. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J. Gastrointest. Liver Dis. 2012, 21, 187–196. [Google Scholar]
- Cell Culture Guides. Available online: https://www.atcc.org/~/media/PDFs/Culture%20Guides/Cell_Lines_by_Gene_Mutation.ashx (accessed on 03 June 2017).
- Fernández, B.; Oyarzabal, I.; Fischer-Fodor, E.; Macavei, S.; Sánchez, I.; Seco, J.M.; Gómez-Ruiz, S.; Rodríguez-Diéguez, A. Multifunctional applications of a dysprosium-based metal–organic chain with single-ion magnet behaviour. CrystEngComm 2016, 18, 8718–8721. [Google Scholar] [CrossRef]











| Material | Theoretical wt % Ti | Experimental wt % Ti 1 | Experimental mmol of Ti/g Material |
|---|---|---|---|
| S1 | 7 | 1.41 | 0.869 |
| S2 | 7 | 1.32 | 0.869 |
| S3 | 7 | 1.33 | 0.869 |
| S4 | 7 | 6.47 | 1.739 |
| Material | SBA-15 | S1 | S2 | S3 | S4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Miller index | 100 | 110 | 100 | 110 | 100 | 110 | 100 | 110 | 100 | 110 |
| Interplanar distance (Å) | 73.59 | 14.77 | 74.33 | 14.83 | 72.09 | 14.71 | 73.37 | 14.71 | 74.56 | 14.94 |
| 2θ (°) | 0.93 | 1.84 | 0.92 | 1.83 | 0.95 | 1.85 | 0.93 | 1.83 | 0.91 | 1.81 |
| Materials | SBET | Total Pore Volume | DP (BJH) 1 | d100 | Wall Thickness 2 | VP |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (Å) | (Å) | (Å) | (cm3/g) | |
| SBA-15 | 857 | 0.88 | 71.5 | 73.6 | 13.5 | 0.88 |
| S1 | 792 | 0.83 | 69.8 | 74.33 | 16.02 | 0.83 |
| S2 | 850 | 0.90 | 42.1 | 72.09 | 41.14 | 0.90 |
| S3 | 793 | 0.85 | 42.9 | 73.37 | 41.82 | 0.85 |
| S4 | 520 | 0.59 | 46.1 | 74.56 | 39.99 | 0.59 |
| Cell Line | EC50 [μg/mL] | ||||
|---|---|---|---|---|---|
| Material/Compound | |||||
| S1 | S2 | S3 | S4 | Oxaliplatin | |
| HepG2 | 613.3 ± 29.8 | 769.7 ± 25.7 | 556.8 ± 40.9 | 403.3 ± 20.8 | 338.9 ± 11.6 |
| Hep3B | 298.0 ±15.7 | 145.3 ± 27.2 | 78.9 ± 4.2 | 66.9 ± 3.9 | 96.1 ± 12.4 |
| SK-Hep-1 | 235.3 ± 26.2 | 272.7 ± 38.4 | 95.5 ± 8.9 | 79.5± 9.3 | 68.3 ± 8.0 |
| DLD-1 | 438.6 ± 39.1 | 227.4 ± 16.2 | 157.5 ± 25.7 | 94.2 ± 10.3 | 142.5 ± 45.9 [33] |
| HT-29 | 155.3 ± 18.4 | 165.7 ± 12.3 | 129.1 ± 10.8 | 85.6 ± 5.8 | 209.0 ± 13.2 [33] |
| COLO320 | 662.0 ± 21.2 | 470.8 ± 34.9 | 306.8 ± 9.7 | 393.4 ± 12.7 | 88.6 ± 0.5 |
| LIV | 757.2 ± 193.2 | >1000 | >1000 | >1000 | 217.1± 6.9 |
| BJ | >1000 | >1000 | 958.0 ± 168.8 | 658.5 ± 231.6 | 252.4 ± 11.8 |
| Cell Line | Deviation from 0 | Material S1 | Material S2 | Material S3 | Material S4 |
|---|---|---|---|---|---|
| HepG2 | Hillslope | −749.6 ± 481.2 | −352.2 ± 292.4 | −534.3 ± 425.7 | −1066 ± 328.2 |
| p value | 0.1416 | 0.2483 | 0.2299 | 0.0058 | |
| Hep3B | Hillslope | −1048 ± 145.2 | −1267 ± 23.2 | −1436 ± 167.0 | −1462 ± 185.3 |
| p value | < 0.0001 | < 0.0001 | < 0.0001 | 0.0002 | |
| SK-Hep-1 | Hillslope | −1215 ± 236.1 | −1326 ± 234.2 | −1876 ± 358.2 | −1116 ± 308.6 |
| p value | 0.0001 | < 0.0001 | 0.0001 | 0.0028 | |
| DLD-1 | Hillslope | −360.9 ± 209.1 | −719.3 ± 129.1 | −916.2 ± 216.6 | −1400 ± 319.7 |
| p value | 0.0610 | 0.0063 | 0.0106 | 0.0005 | |
| HT-29 | Hillslope | −1082 ± 278.3 | −1066 ± 286.3 | −2063 ± 277.8 | −1263 ± 212.1 |
| p value | 0.0013 | 0.0019 | < 0.0001 | < 0.0001 | |
| COLO320 | Hillslope | −369.0 ± 227.8 | −278.8 ± 188.4 | −683.7 ± 112.0 | −1195 ± 236.5 |
| p value | 0.1276 | 0.1612 | < 0.0001 | 0.0002 |
| t (h) | wt % Ti in S1 | Release (%) in S1 | wt % Ti in S4 | Release (%) in S4 |
|---|---|---|---|---|
| 0 | 1.26 | 0 | 6.47 | 0 |
| 1 | 1.12 | 11 | 6.10 | 6 |
| 6 | 1.14 | 9 | 5.83 | 10 |
| 24 | 1.18 | 6 | 5.12 | 21 |
| 48 | 1.21 | 4 | 4.77 | 26 |
| 96 | 1.19 | 5 | 4.48 | 31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. https://doi.org/10.3390/ma11020224
Gómez-Ruiz S, García-Peñas A, Prashar S, Rodríguez-Diéguez A, Fischer-Fodor E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials. 2018; 11(2):224. https://doi.org/10.3390/ma11020224
Chicago/Turabian StyleGómez-Ruiz, Santiago, Alberto García-Peñas, Sanjiv Prashar, Antonio Rodríguez-Diéguez, and Eva Fischer-Fodor. 2018. "Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation" Materials 11, no. 2: 224. https://doi.org/10.3390/ma11020224
APA StyleGómez-Ruiz, S., García-Peñas, A., Prashar, S., Rodríguez-Diéguez, A., & Fischer-Fodor, E. (2018). Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials, 11(2), 224. https://doi.org/10.3390/ma11020224