Stepwise Thermo-Responsive Amino Acid-Derived Triblock Vinyl Polymers: ATRP Synthesis of Polymers, Aggregation, and Gelation Properties via Flower-Like Micelle Formation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of ABA-Type Triblock Copolymers from Amino Acid–Based Vinyl Monomers
2.2. Thermal Properties of Poly(NAAMen-b-NAGMe240-b-NAAMen) in Water at a Diluted Condition
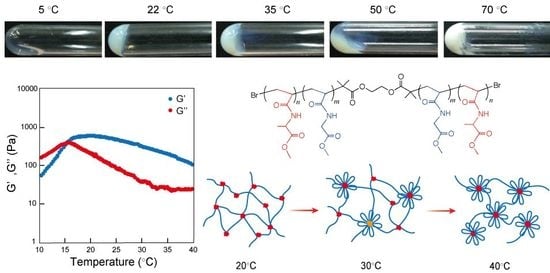
2.3. Self-Assembly of Triblock Copolymer in Concentrated Aqueous Solutions—Gelation and Injectable Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Bromine-Terminated Poly(NAGMe) Macro-Initiator
3.3. Synthesis of Triblock Copolymers (Poly(NAAMen-b-PNAGMe240-b-NAAMen))
3.4. Methods and Procedures
3.4.1. Spectroscopies
3.4.2. Size Exclusion Chromatography (SEC)
3.4.3. MTT Assay
3.4.4. Microscopic Observations
3.4.5. Dynamic Light Scattering (DLS)
3.4.6. Rheology Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galaev, I.; Mattiasson, B. Smart Polymers: Applications in Biotechnology and Biomedicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Yin, X.; Stayton, P.S.; Hoffman, A.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Convertine, A.J.; Ayres, N.; Scales, C.W.; Lowe, A.B.; McCormick, C.L. Facile, Controlled, Room-Temperature RAFT Polymerization of N-Isopropylacrylamide. Biomacromolecules 2004, 5, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of N-isopropylacrylamide and poly(N-isopropylacrylamide)-coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Akdemir, O.; Hoth, A. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of poly(NIPAM) over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Higashi, N.; Sonoda, R.; Koga, T. Thermo-responsive amino acid-based vinyl polymers showing widely tunable LCST/UCST behavior in water. RSC Adv. 2015, 5, 67652–67657. [Google Scholar] [CrossRef]
- Mori, H.; Iwaya, H.; Nagai, A.; Endo, T. Controlled synthesis of thermoresponsive polymers derived from l-proline via RAFT polymerization. Chem. Commun. 2005, 4872–4874. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Non-ionic homo-and copolymers with H-donor and H-acceptor units with an UCST in water. Macromol. Chem. Phys. 2010, 211, 2109–2117. [Google Scholar] [CrossRef]
- Shimada, N.; Ino, H.; Maie, K.; Nakayama, M.; Kano, A.; Maruyama, A. Ureido-derivatized polymers based on both poly(allylurea) and poly(l-citrulline) exhibit UCST-type phase transition behavior under physiologically relevant conditions. Biomacromolecules 2011, 12, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, G.; Ma, Y.; Yu, D.; Sun, J.; Li, Z. Smart surfaces based on thermo-responsive polymer brushes prepared from L-alanine derivatives for cell capture and release. Soft Matter 2015, 11, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Higashi, N.; Hirata, A.; Nishimura, S.; Koga, T. Thermo-responsive polymer brushes on glass plate prepared from a new class of amino acid-derived vinyl monomers and their applications in cell-sheet engineering. Colloid Surf. B Biointerfaces 2017, 159, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.C.; Roux, E.; Le Garrec, D.; Hong, K.L.; Drummond, D.C. N-Isopropylacrylamide copolymers for the preparation of pH-sensitive liposomes and polymeric micelles. J. Control. Release 2001, 72, 71–84. [Google Scholar] [CrossRef]
- Feul, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Semenov, A.N.; Joanny, J.F.; Khokhlov, A.R. Associating polymers: Equilibrium and linear viscoelasticity. Macromolecules 1995, 28, 1066–1075. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Nah, J.W.; Lee, H.C.; Kim, S.H.; Cho, C.S. Adriamycin release from flower-type polymeric micelle based on star-block copolymer composed of poly(γ-benzyl l-glutamate) as the hydrophobic part and poly(ethylene oxide) as the hydrophilic part. Int. J. Pharm. 1999, 188, 49–58. [Google Scholar] [CrossRef]
- Konak, C.; Helmstedt, M.; Bansil, R. Temperature dependence of dynamics of solutions of triblock copolymer in a selective solvent. Polymer 2000, 41, 9311–9315. [Google Scholar] [CrossRef]
- Resendes, R.; Massey, J.A.; Temple, K.; Cao, L.; Power-Billard, K.N.; Winnik, M.A.; Manners, I. Supramolecular organometallic polymer chemistry: Multiple morphologies and superstructures from the solution self-assembly of polyferrocene-block-polysiloxane-block-polyferrocene triblock copolymers. Chem. Eur. J. 2001, 7, 2414–2424. [Google Scholar] [CrossRef]
- Tanaka, F. Intramolecular micelles and intermolecular crosslinks in thermo-reversible gels of associating polymers. J. Non-Cryst. Solids 2002, 307, 688–697. [Google Scholar] [CrossRef]
- Liu, T.; Kim, K.; Hsiao, B.S.; Chu, B. Regular and irregular micelles formed by A LEL triblock copolymer in aqueous solution. Polymer 2004, 45, 7989–7993. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsiue, G.-H. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J. Control. Release 2005, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Hamley, I.W.; Ma, Y.; Bories-Azeau, X.; Armes, S.P.; Lewis, A.L. Microstructure and physical properties of a pH-responsive gel based on a novel biocompatible ABA-type triblock copolymer. Langmuir 2004, 20, 4306–4309. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Higashi, N.; Sekine, D.; Koga, T. Temperature induced self-assembly of amino acid-drived vinyl block copolymers via dual phase transitions. J. Colloid Interface Sci. 2017, 500, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashi, N.; Matsubara, S.; Nishimura, S.-n.; Koga, T. Stepwise Thermo-Responsive Amino Acid-Derived Triblock Vinyl Polymers: ATRP Synthesis of Polymers, Aggregation, and Gelation Properties via Flower-Like Micelle Formation. Materials 2018, 11, 424. https://doi.org/10.3390/ma11030424
Higashi N, Matsubara S, Nishimura S-n, Koga T. Stepwise Thermo-Responsive Amino Acid-Derived Triblock Vinyl Polymers: ATRP Synthesis of Polymers, Aggregation, and Gelation Properties via Flower-Like Micelle Formation. Materials. 2018; 11(3):424. https://doi.org/10.3390/ma11030424
Chicago/Turabian StyleHigashi, Nobuyuki, Sho Matsubara, Shin-nosuke Nishimura, and Tomoyuki Koga. 2018. "Stepwise Thermo-Responsive Amino Acid-Derived Triblock Vinyl Polymers: ATRP Synthesis of Polymers, Aggregation, and Gelation Properties via Flower-Like Micelle Formation" Materials 11, no. 3: 424. https://doi.org/10.3390/ma11030424
APA StyleHigashi, N., Matsubara, S., Nishimura, S. -n., & Koga, T. (2018). Stepwise Thermo-Responsive Amino Acid-Derived Triblock Vinyl Polymers: ATRP Synthesis of Polymers, Aggregation, and Gelation Properties via Flower-Like Micelle Formation. Materials, 11(3), 424. https://doi.org/10.3390/ma11030424