Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Injectable Bone Substitutes (IBS) Samples
2.2.1. Preparation of the Polymeric Solution
2.2.2. Preparation of the Bioceramic Powder Mixture
2.3. Characterization of IBS Samples
2.3.1. XRD Analysis
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3.3. Injectability Measurements
2.3.4. SEM Analysis
2.3.5. Rheological Measurements
2.3.6. Compressive Strength Measurements
2.3.7. pH Changes
2.3.8. In Vitro Degradation
3. Results and Discussion
3.1. Analysis of the Synthesized Powder
3.2. Injectability of IBS Samples
3.3. Morphology of the IBS Samples
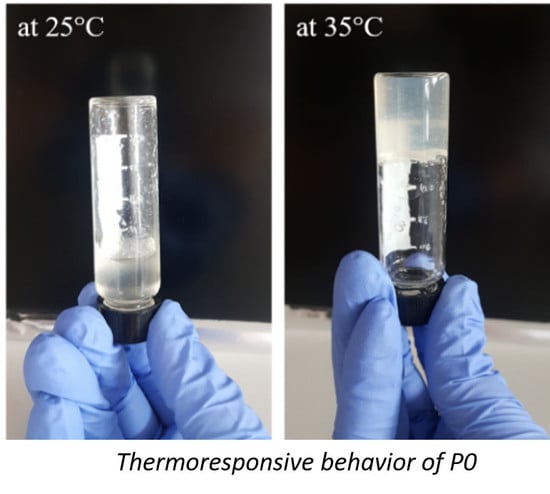
3.4. Rheological Measurements
3.5. pH Change
3.6. In Vitro Degradation
3.7. XRD Analysis
3.8. Compressive Strength Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown,, E.; Chow, L.C. A New Calcium Phosphate, Setting Cement. J. Dent. 1983, 62, 672. [Google Scholar]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Thai, V.V.; Lee, B.T. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J. Mater. Sci. Mater. Med. 2010, 21, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Rangabhatla, A.S.L.; Tantishaiyakul, V.; Oungbho, K.; Boonrat, O. Fabrication of pluronic and methylcellulose for etidronate delivery and their application for osteogenesis. Int. J. Pharm. 2016, 499, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Kang, L.; Xu, F.; Wang, Z.; Cui, F.-Z.; Guo, Z. Recent progress in injectable bone repair materials research. Front. Mater. Sci. 2015, 9, 332–345. [Google Scholar] [CrossRef]
- Perez, R.A.; Shin, S.-H.; Han, C.-M.; Kim, H.-W. Bioactive injectables based on calcium phosphates for hard tissues: A recent update. Tissue Eng. Regen. Med. 2015, 12, 143–153. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Li, C.; Weir, M.D.; Wang, P.; Reynolds, M.A.; Zhao, L.; Xu, H.H.K. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater. Sci. Eng. C 2016, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.V.; Sivshanmugam, A.; Boccaccini, A.R.; Goudouri, O.M.; Sun, W.; Hwang, N.; Deepthi, S.; Nair, S.V.; Jayakumar, R. Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef]
- Shimokawa, K.; Saegusa, K.; Ishii, F. Rheological properties of reversible thermo-setting in situ gelling solutions with the methylcellulose-polyethylene glycol-citric acid ternary system (2): Effects of various water-soluble polymers and salts on the gelling temperature. Colloid Surf. B Biointerfaces 2009, 74, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Basnett, P.; Knowles, J.C.; Pishbin, F.; Smith, C.; Keshavarz, T.; Boccaccini, A.R.; Roy, I. Novel biodegradable and biocompatible poly(3-hydroxyoctanoate)/bacterial cellulose composites. Adv. Eng. Mater. 2012, 14, 330–343. [Google Scholar] [CrossRef]
- Perale, G.; Rossi, F.; Santoro, M.; Peviani, M.; Papa, S.; Llupi, D.; Torriani, P.; Micotti, E.; Previdi, S.; Cervo, L.; et al. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J. Control. Release 2012, 159, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.K.; Maity, D.; Bhowmick, B.; Mondal, D.; Mollick, M.M.R.; Sarkar, G.; Bhowmik, M.; Rana, D.; Chattopadhyay, D. Effect of PEG-salt mixture on the gelation temperature and morphology of MC gel for sustained delivery of drug. Carbohydr. Polym. 2013, 91, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Park, J.; Yoo, K.; Kim, W.; Kim, D.H.; Yoon, S.Y. Preparation, characterization, and in-vitro performance of novel injectable silanized-hydroxypropyl methylcellulose/phase-transformed calcium phosphate composite bone cements. Curr. Appl. Phys. 2016, 16, 1523–1532. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Hilbig, U.; Hoffmann, C.; Unger, R.E.; Sader, R.A.; Peters, F.; Kirkpatrick, C.J. An injectable bone substitute composed of beta-tricalcium phosphate granules, methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomater. 2011, 7, 4018–4028. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Oheim, R.; Catala-Lehnen, P.; Pestka, J.M.; Hoffmann, C.; Huebner, W.; Peters, F.; Barvencik, F.; Amling, M. Metaphyseal bone formation induced by a new injectable beta-TCP-based bone substitute: A controlled study in rabbits. J. Biomater. Appl. 2014, 28, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, M.; Hoare, T. Injectable, mixed natural-synthetic polymer hydrogels with modular properties. Biomacromolecules 2012, 13, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, P. Injectable thermo-responsive hydrogel composed of xanthan gum and methylcellulose double networks with shear-thinning property. Carbohydr. Polym. 2015, 132, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Félix Lanao, R.P.; Sariibrahimoglu, K.; Wang, H.; Wolke, J.G.C.; Jansen, J.A.; Leeuwenburgh, S.C.G. Accelerated calcium phosphate cement degradation due to incorporation of glucono-delta-lactone microparticles. Tissue Eng. Part A 2014, 20, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Dessì, M.; Alvarez-Perez, M.A.; De Santis, R.; Ginebra, M.P.; Planell, J.A.; Ambrosio, L. Bioactivation of calcium deficient hydroxyapatite with foamed gelatin gel. A new injectable self-setting bone analogue. J. Mater. Sci. Mater. Med. 2014, 25, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Utech, S.; Boccaccini, A.R. A review of hydrogel-based composites for biomedical applications: enhancement of hydrogel properties by addition of rigid inorganic fillers. J. Mater. Sci. 2016, 51, 271–310. [Google Scholar] [CrossRef]
- Bongio, M.; Nejadnik, M.R.; Kasper, F.K.; Mikos, A.G.; Jansen, J.A.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P. Development of an in vitro confinement test to predict the clinical handling of polymer-based injectable bone substitutes. Polym. Test. 2013, 32, 1379–1384. [Google Scholar] [CrossRef]
- Nishinari, K.; Hofmann, K.E.; Kohyama, K.; Moritaka, H.; Nishinari, N.; Watase, M. Polysaccharide-protein interaction: A rheological study of the gel-sol transition of a gelatin-methylcellulose-water system. Biorheology 1993, 30, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Jonge, L.T.; De Wolke, J.G.C.; Yubao, L.; Mikos, A.G.; Jansen, J.A. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J. Biomed. Mater. Res. A 2008, 87, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín-Masiá, E.; Poveda-Reyes, S.; Gallego Ferrer, G. Extracellular matrix–inspired gelatin/hyaluronic acid injectable hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 280–288. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, Y.; Lei, M.; Du, Y.; Kennedy, J.F.; Knill, C.J. Production and characterisation of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr. Polym. 2010, 82, 833–841. [Google Scholar] [CrossRef]
- Sadiasa, A.; Sarkar, S.K.; Franco, R.A.; Min, Y.K.; Lee, B.T. Bioactive glass incorporation in calcium phosphate cement-based injectable bone substitute for improved in vitro biocompatibility and in vivo bone regeneration. J. Biomater. Appl. 2013, 28, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Hempel, U.; Reinstorf, A.; Poppe, M.; Fischer, U.; Gelinsky, M.; Pompe, W.; Wenzel, K.W. Proliferation and Differentiation of Osteoblasts on Biocement D Modified with Collagen Type I and Citric Acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71B, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, J.; Wang, H. Effects of Additives on the Rheological Properties and Injectability of a Calcium Phosphate Bone Substitute Material. J. Biomed. Mater. Res. Part B Appl. Polym. 2005, 78B, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, K.; Han, Y. Influence of cooling modes on purity of solid-state synthesized tetracalcium phosphate. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2005, 116, 175–181. [Google Scholar] [CrossRef]
- Song, H.Y.; Rahman, A.H.M.E.; Lee, B.T. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using chitosan and citric acid. J. Mater. Sci. Mater. Med. 2009, 20, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.L.R.; dos Santos, L.A.; Bergmann, C.P. Injectability evaluation of tricalcium phosphate bone cement. J. Mater. Sci. Mater. Med. 2008, 19, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.R.; Deng, X.H.; Zhou, L.X.; Gao, X.; Fan, M.; Wang, Y.L.; Guo, G. Injectable thermosensitive hydrogel composite with surface-functionalized calcium phosphate as raw materials. Int. J. Nanomed. 2014, 9, 615–626. [Google Scholar] [CrossRef]
- Yokoyama, A.; Yamamoto, S.; Kawasaki, T.; Kohgo, T.; Nakasu, M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials 2002, 23, 1091–1101. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, Q.; Yu, B.; Li, S. Biomimetic properties of an injectable chitosan / nano-hydroxyapatite / collagen composite. Mater. Sci. Eng. C 2011, 31, 683–687. [Google Scholar] [CrossRef]
- Radwan, M.M.; Abd El-Hamid, H.K.; Nagi, S.M. Synthesis, properties and hydration characteristics of novel nano-size mineral trioxide and tetracalcium phosphate for dental applications. Orient. J. Chem. 2016, 32, 2459–2472. [Google Scholar] [CrossRef]
- Jayasree, R.; Kumar, T.S.; Kavya, K.P.S.; Nankar, P.R.; Mukesh, D. Self Setting Bone Cement Formulations Based on Egg shell Derived TetraCalcium Phosphate BioCeramics. Bioceram. Dev. Appl. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Liao, J.; Duan, X.; Li, Y.; Zheng, C.; Yang, Z.; Zhou, A.; Zou, D. Synthesis and mechanism of tetracalcium phosphate from nanocrystalline precursor. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Kim, H.; Camata, R.P.; Vohra, Y.K.; Lacefield, W.R. Control of phase composition in hydroxyapatite/tetracalcium phosphate biphasic thin coatings for biomedical applications. J. Mater. Sci. Mater. Med. 2005, 16, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Solati-Hashjin, M.; Tahriri, M. Synthesis and Characterization of Hydroxyapatite Nanocrystals via Chemical Precipitation Technique. Iran. J. Pharm. Sci. 2008, 4, 127–134. [Google Scholar]
- Liu, W.; Zhang, J.; Rethore, G.; Khairoun, K.; Pilet, P.; Tancret, F.; Bouler, J.M.; Weiss, P. A novel injectable, cohesive and toughened Si-HPMC (silanized-hydroxypropyl methylcellulose) composite calcium phosphate cement for bone substitution. Acta Biomater. 2014, 10, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Marefat Seyedlar, R.; Nodehi, A.; Atai, M.; Imani, M. Gelation behavior of in situ forming gels based on HPMC and biphasic calcium phosphate nanoparticles. Carbohydr. Polym. 2014, 99, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Ai, J.; Nourani, M.R.; Azami, M.; Hashemi Beni, B.; Asadpour, S.; Bordbar, S. Injectable natural polymer compound for tissue engineering of intervertebral disc: In vitro study. Mater. Sci. Eng. C 2017, 80, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, S.A.; Lott, J.R.; McAllister, J.W.; Zhang, J.; Bates, F.S.; Lodge, T.P.; Sammler, R.L.; Li, Y.; Brackhagen, M. Interplay of phase separation and thermoreversible gelation in aqueous methylcellulose solutions. Macromolecules 2013, 46, 300–309. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Shi, Y.; Wan, Y. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/hydroxyapatite/glycerophosphate hydrogels. J. Mech. Behav. Biomed. Mater. 2016, 64, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Tator, C.H.; Shoichet, M.S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006, 27, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.C.; Shear, D.A.; Hoffman, S.W.; Stein, D.G.; LaPlaca, M.C. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials 2001, 22, 1113–1123. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Fan, D.; Xue, W.; Zhu, C.; Li, X.; Liu, Y.; Liu, W.; Ma, P.; Wang, Y. Physicochemical properties and biological behavior of injectable crosslinked hydrogels composed of pullulan and recombinant human-like collagen. J. Mater. Sci. 2017, 52, 3771–3785. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, S.; Yu, B.; Yan, Y.; Li, H.; Wei, J.; Su, J. In vitro degradability, bioactivity and primary cell responses to bone cements containing mesoporous magnesium–calcium silicate and calcium sulfate for bone regeneration. J. R. Soc. Interface 2015, 12, 20150779. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Husain, S.; Huang, Y.; Pogorielov, M.; Deineka, V.; Lyndin, M.; Rawlinson, A.; Rehman, I.U. In-vitro and in-vivo degradation studies of freeze gelated porous chitosan composite scaffolds for tissue engineering applications. Polym. Degrad. Stab. 2017, 136, 31–38. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Baino, F. Ceramics for bone replacement. In Advances in Ceramic Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 249–278. ISBN 9780081008812. [Google Scholar]
- Ege, D.; Kamali, A.R.; Boccaccini, A.R. Graphene Oxide/Polymer-Based Biomaterials. Adv. Eng. Mater. 2017, 19. [Google Scholar] [CrossRef]
- Newman, P.; Minett, A.; Ellis-Behnke, R.; Zreiqat, H. Carbon nanotubes: Their potential and pitfalls for bone tissue regeneration and engineering. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1139–1158. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Zein, S.H.S.; Gerhardt, L.-C.; Low, K.L.; Tan, S.H.; McPhail, D.S.; Grover, L.M.; Boccaccini, A.R. Cytocompatibility, bioactivity and mechanical strength of calcium phosphate cement reinforced with multi-walled carbon nanotubes and bovine serum albumin. Ceram. Int. 2013, 39, 4975–4983. [Google Scholar] [CrossRef]














| Abbreviation | Gelatin (wt %) | SC (wt %) | MC (wt %) | Bioceramic Powder Component (wt %) |
|---|---|---|---|---|
| P0 | 2.5 | 3 | 8 | 0 |
| P20 | 2.5 | 3 | 8 | 20 |
| P30 | 2.5 | 3 | 8 | 30 |
| P50 | 2.5 | 3 | 8 | 50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir Oğuz, Ö.; Ege, D. Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials 2018, 11, 604. https://doi.org/10.3390/ma11040604
Demir Oğuz Ö, Ege D. Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials. 2018; 11(4):604. https://doi.org/10.3390/ma11040604
Chicago/Turabian StyleDemir Oğuz, Öznur, and Duygu Ege. 2018. "Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes" Materials 11, no. 4: 604. https://doi.org/10.3390/ma11040604
APA StyleDemir Oğuz, Ö., & Ege, D. (2018). Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials, 11(4), 604. https://doi.org/10.3390/ma11040604