Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Degree of Conversion (DC)
2.3. Flexural Strength and Flexural Modulus
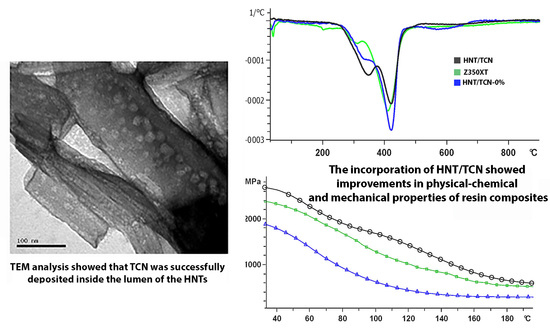
2.4. Dynamic Thermomechanical Analysis (DMA)
2.5. Thermogravimetric Analysis (TGA)
2.6. Polymerization Stress Measurements (PS)
2.7. Microbiology Assay
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Incorporation of 8 wt.% seems to be a satisfactory formulation of halloysite nanotube for achieving appropriate mechanical properties for an experimental resin micro-hybrid composite without affecting the viscosity and the material and increase the risk for phase separation;
- The experimental resin composite containing 8 wt.% halloysite nanotube doped with triclosan, from a physicochemical point of view, seems to be a suitable restorative material such as the current commercial nano-filled resin-composite;
- Incorporation of triclosan seems to be test-dependent, since it showed no response in mature biofilms as used in this study.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in dental materials through nanotechnology: Facts, perspectives and toxicological aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Q.; Zhang, X.; Wei, Y.; Xu, M.; Yang, X.; Ma, Q.; Cheng, Y.; Deng, X. Improved performance of Bis-GMA/TEGDMA dental composites by net-like structures formed from SiO2 nanofiber fillers. Mater. Sci. Eng. C 2016, 59, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Leitune, V.C.B.; Takimi, A.S.; Collares, F.M.; Sauro, S. Physicochemical and bioactive properties of innovative resin-based materials containing functional halloysite-nanotubes fillers. Dent. Mater. 2016, 32, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Luis, H.; Martin, M.D.; Leroux, B.G.; Rue, T.; Leitão, J.; DeRouen, T.A. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J. Am. Dent. Assoc. 2007, 138, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Corrêa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J.M. Longevity of posterior composite restorations: Not only a matter of materials. Dent. Mater. 2012, 28, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loesche, W.J. Role of streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [PubMed]
- Fugolin, A.P.P.; Pfeifer, C.S. New resins for dental composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-X.; Tan, L.; Tang, Z.-W.; Yang, M.-Y.; Xiao, J.-Y.; Liu, C.-J.; Zhuo, R.-X. Highly efficient antibacterial surface grafted with a triclosan-decorated poly(n-hydroxyethylacrylamide) brush. ACS Appl. Mater. Interfaces 2015, 7, 7008–7015. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Genari, B.; Leitune, V.C.B.; Arthur, R.A.; Luxan, S.A.; Samuel, S.M.W.; Collares, F.M.; Sauro, S. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J. Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kaffashi, B.; Davoodi, S.; Oliaei, E. Poly(ϵ-caprolactone)/triclosan loaded polylactic acid nanoparticles composite: A long-term antibacterial bionanocomposite with sustained release. Int. J. Pharm. 2016, 508, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rathke, A.; Staude, R.; Muche, R.; Haller, B. Antibacterial activity of a triclosan-containing resin composite matrix against three common oral bacteria. J. Mater. Sci. Mater. Med. 2010, 21, 2971–2977. [Google Scholar] [CrossRef] [PubMed]
- Wicht, M.; Haak, R.; Kneist, S.; Noack, M. A triclosan-containing compomer reduces spp. predominant in advanced carious lesions. Dent. Mater. 2005, 21, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, S.A.; Münchow, E.A.; Al-Zain, A.O.; Kamocki, K.; Platt, J.A.; Bottino, M.C. Synthesis and characterization of novel halloysite-incorporated adhesive resins. J. Dent. 2015, 43, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J. Phys. Chem. C 2010, 114, 11358–11363. [Google Scholar] [CrossRef]
- Bottino, M.C.; Batarseh, G.; Palasuk, J.; Alkatheeri, M.S.; Windsor, L.J.; Platt, J.A. Nanotube-modified dentin adhesive—Physicochemical and dentin bonding characterizations. Dent. Mater. 2013, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite nanotubes for cleaning, consolidation and protection. Chem. Rec. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alkatheeri, M.S.; Palasuk, J.; Eckert, G.J.; Platt, J.A.; Bottino, M.C. Halloysite nanotube incorporation into adhesive systems—Effect on bond strength to human dentin. Clin. Oral Investig. 2015, 19, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi-Ghahfarrokhi, M.; Haddadi-Asl, V.; Zargarian, S.S. Fabrication and characterization of polymer-ceramic nanocomposites containing drug loaded modified halloysite nanotubes. J. Biomed. Mater. Res. Part A 2018. [Google Scholar] [CrossRef] [PubMed]
- Palasuk, J.; Windsor, L.J.; Platt, J.A.; Lvov, Y.; Geraldeli, S.; Bottino, M.C. Doxycycline-loaded nanotube-modified adhesives inhibit MMP in a dose-dependent fashion. Clin. Oral Investig. 2017. [CrossRef] [PubMed]
- Feitosa, S.A.; Palasuk, J.; Kamocki, K.; Geraldeli, S.; Gregory, R.L.; Platt, J.A.; Windsor, L.J.; Bottino, M.C. Doxycycline-encapsulated nanotube-modified dentin adhesives. J. Dent. Res. 2014, 93, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Svizero Nda, R.; Silva, M.S.; Alonso, R.C.; Rodrigues, F.P.; Hipólito, V.D.; Carvalho, R.M.; D’Alpino, P.H. Effects of curing protocols on fluid kinetics and hardness of resin cements. Dent. Mater. J. 2013, 32, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, N.S.; de Souza, L.C.; Feitosa, V.P.; Loguercio, A.D.; D’Arcangelo, C.; Sauro, S.; Saboia, V.d.P.A. Effect of different conditioning/deproteinization protocols on the bond strength and degree of conversion of self-adhesive resin cements applied to dentin. Int. J. Adhes. Adhes. 2017. [Google Scholar] [CrossRef]
- ISO 4049. Dentistry—Polymer-Based Filling, Restorative and Luting Materials; 7.10 Depth of cure, Class 2 materials; International Organization for Standardization: Geneva,Switzerland, 2000. [Google Scholar]
- Gonçalves, F.; Boaro, L.C.; Ferracane, J.L.; Braga, R.R. A comparative evaluation of polymerization stress data obtained with four different mechanical testing systems. Dent. Mater. 2012, 28, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Schaich, K.; Bowen, W.H.; Koo, H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.L.; Carvalho, P.H.A.; van de Sande, F.H.; Pereira, C.M.P.; Piva, E.; Lund, R.G. Self-etching dental adhesive containing a natural essential oil: Anti-biofouling performance and mechanical properties. Biofouling 2013, 29, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, Y.; Wu, W.; Xu, T.; Fong, H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing halloysite nanotubes. Dent. Mater. 2012, 28, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abzal, M.; Rathakrishnan, M.; Prakash, V.; Vivekanandhan, P.; Subbiya, A.; Sukumaran, V. Evaluation of surface roughness of three different composite resins with three different polishing systems. J. Conserv. Dent. 2016, 19. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Sen, A. Rate enhancement in controlled radical polymerization of acrylates using recyclable heterogeneous lewis acid. Macromolecules 2007, 40, 154–156. [Google Scholar] [CrossRef]
- Karabela, M.M.; Sideridou, I.D. Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent. Mater. 2011, 27, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, A.; Yih, J.A.; Platta, J.; Knight, J.; Pfeifer, C.S. Shrinkage/stress reduction and mechanical properties improvement in restorative composites formulated with thio-urethane oligomers. J. Mech. Behav. Biomed. Mater. 2018, 78, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Aydınoğlu, A.; Yoruç, A.B.H. Effects of silane-modified fillers on properties of dental composite resin. Mater. Sci. Eng. C 2017, 79, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Boaro, L.C.C.; Gonalves, F.; Guimarães, T.C.; Ferracane, J.L.; Versluis, A.; Braga, R.R. Polymerization stress, shrinkage and elastic modulus of current low-shrinkage restorative composites. Dent. Mater. 2010, 26, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.S.; Balbinot, C.E.; Blando, E.; Mota, E.G.; Oshima, H.M.; Hirakata, L.; Pires, L.A.; Hübler, R. Evaluation of mechanical properties on three nanofilled composites. Stomatologija 2012, 14, 126–130. [Google Scholar] [PubMed]
- Gonçalves, F.; Boaro, L.C.C.; Miyazaki, C.L.; Kawano, Y.; Braga, R.R. Influence of polymeric matrix on the physical and chemical properties of experimental composites. Braz. Oral Res. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boaro, L.C.C.; Fróes-Salgado, N.R.; Gajewski, V.E.S.; Bicalho, A.A.; Valdivia, A.D.C.M.; Soares, C.J.; Miranda Júnior, W.G.; Braga, R.R. Correlation between polymerization stress and interfacial integrity of composites restorations assessed by different in vitro tests. Dent. Mater. 2014, 30, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.P.; Lima, R.G.; Muench, A.; Watts, D.C.; Ballester, R.Y. A method for calculating the compliance of bonded-interfaces under shrinkage: Validation for Class i cavities. Dent. Mater. 2014, 30, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Labella, R.; Lambrechts, P.; Van Meerbeek, B.V.G. Polymerization shrinkage and elasticity of flowable adhesives and filled composites. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Rosatto, C.M.P.; Bicalho, A.A.; VerÃssimo, C.; Bragança, G.F.; Rodrigues, M.P.; Tantbirojn, D.; Versluis, A.; Soares, C.J. Mechanical properties, shrinkage stress, cuspal strain and fracture resistance of molars restored with bulk-fill composites and incremental filling technique. J. Dent. 2015, 43, 1519–1528. [Google Scholar] [CrossRef] [PubMed]



| Name of the Composite | Type of Composite | Manufacturer/Lot No. | Composition |
|---|---|---|---|
| HNT/TCN-0% | Experimental resin-composite | ----- | Organic matrix: Bis-GMA, TEGDMA. |
| Filler type: Silica micro-hybrid filler 80 wt.% | |||
| Filler content: 80 wt.% | |||
| HNT/TCN | Experimental resin-composite | ----- | Organic matrix: Bis-GMA, TEGDMA. |
| Filler type: Silica micro-hydrid filler 72 wt.% and halloysite nanotubes 8 wt.%. | |||
| Filler content: 80 wt.% | |||
| Filtek Z-350XT (Shade A1D) | Commercial nano-filled composite | 3M ESPE (St Paul, MN, USA)/N702257 | Organic matrix: Bis-GMA, Bis-EMA, UDMA, TEGDMA |
| Filler type: Silica and zirconia nanofillers, agglomerated zirconia-silica nanoclusters | |||
| Filler content: 82 wt.% |
| Composite | DC (%) | E (GPa) | FS (MPa) | PS (MPa) |
|---|---|---|---|---|
| HNT/TCN-0% | 75.9 (5.4) A | 6.8 (0.9) A | 75.9 (10.1) B | 3.6 (0.3) B |
| HNT/TCN | 78.5 (2.2) A | 7.5 (0.2) A | 107.2 (6.6) A | 5.4 (0.9) A |
| Z350XT | 72.5 (10.6) A | 6.8 (0.4) A | 101.4 (18.4) A | 3.6 (0.3) B |
| Composite | Tg (°C) | Tanδ (×103) at Tg | TGA Weight Loss (%) | TGA Temperature of the First Degradation Step (°C) | TGA Temperature of the Second Degradation Step (°C) |
|---|---|---|---|---|---|
| HNT/TCN-0% | 102 (6.56) B | 156.7 (0.15) A | 27.3 (0.58) A | 301 (1.73) A | 426 (3.6) A |
| HNT/TCN | 154 (4.36) A | 106.7 (0.11) B | 26.3 (0.58) A | 296 (1.0) B | 419 (1.15) A,B |
| Z350XT | 105.3 (3.51) B | 103.3 (0.15) B | 27 (1.0) A | 286 (2.64) C | 415 (5.72) B |
| Composite | (CFU)/mL/mm2 | Dry Weight (g) |
|---|---|---|
| HNT/TCN-0% | 6.9267 (0.35) A | 0.0004 (0.00025) A |
| HNT/TCN | 6.8733 (0.28) A | 0.0008 (0.00082) A |
| Z350XT | 6.8867 (0.28) A | 0.0003 (0.00006) A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, D.A.; Rodrigues, N.S.; Souza, L.C.; Lomonaco, D.; Rodrigues, F.P.; Degrazia, F.W.; Collares, F.M.; Sauro, S.; Saboia, V.P.A. Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes. Materials 2018, 11, 1080. https://doi.org/10.3390/ma11071080
Cunha DA, Rodrigues NS, Souza LC, Lomonaco D, Rodrigues FP, Degrazia FW, Collares FM, Sauro S, Saboia VPA. Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes. Materials. 2018; 11(7):1080. https://doi.org/10.3390/ma11071080
Chicago/Turabian StyleCunha, Diana A., Nara S. Rodrigues, Lidiane C. Souza, Diego Lomonaco, Flávia P. Rodrigues, Felipe W. Degrazia, Fabrício M. Collares, Salvatore Sauro, and Vicente P. A. Saboia. 2018. "Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes" Materials 11, no. 7: 1080. https://doi.org/10.3390/ma11071080
APA StyleCunha, D. A., Rodrigues, N. S., Souza, L. C., Lomonaco, D., Rodrigues, F. P., Degrazia, F. W., Collares, F. M., Sauro, S., & Saboia, V. P. A. (2018). Physicochemical and Microbiological Assessment of an Experimental Composite Doped with Triclosan-Loaded Halloysite Nanotubes. Materials, 11(7), 1080. https://doi.org/10.3390/ma11071080